Effect of synthetic purines and purine nucleosides on [3H]diazepam binding in brain.
S C Sung, M Saneyoshi
Index: Eur. J. Pharmacol. 81(3) , 505-8, (1982)
Full Text: HTML
Abstract
We have compared fifteen synthetic purines and purine nucleosides on their ability to displace [3H]diazepam binding to rat brain membranes. Among these analogs, 6-methylthioguanine was found to be most potent, inhibiting competitively the specific binding of [3H]diazepam with a Ki value of 16 micro M. At a concentration of 50 micro M, 6-methyl-thioguanine increased tha apparent Kd of specific diazepam binding from 4.3 nM to 13.3 nM without affecting the Bmax, nor had it any effect on the non-specific binding. Binding with membrane preparations from developing rat brain was slightly less sensitive to 6-methylthioguanine inhibition than that with membranes prepared from mature brain.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
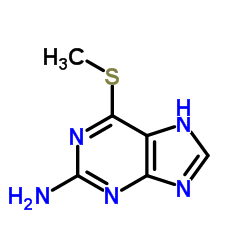 |
Purine, 2-amino-6- (methylthio)
CAS:1198-47-6 |
C6H7N5S |
|
Liquid Chromatography with Amperometric Detection at a Silve...
2015-07-07 [Anal. Chem. 87 , 6730-5, (2015)] |
|
The syntheses and properties of tricyclic pyrrolo[2,3-d]pyri...
2006-05-07 [Org. Biomol. Chem. 4(9) , 1723-9, (2006)] |
|
Reference intervals for thiopurine S-methyltransferase activ...
2004-07-01 [Ann. Clin. Biochem. 41(Pt 4) , 303-8, (2004)] |
|
Effects of various 2-amino-6-alkyldithiopurines on brain spe...
1984-06-01 [Biochem. Pharmacol. 33 , 1737-1739, (1984)] |
|
Role of postreplicative DNA mismatch repair in the cytotoxic...
1996-08-23 [Science 273(5278) , 1109-11, (1996)] |