| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
5-(Benzyloxy)-1-pentanol
CAS:4541-15-5 |
|
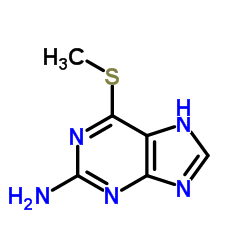 |
Purine, 2-amino-6- (methylthio)
CAS:1198-47-6 |