| Structure | Name/CAS No. | Articles |
|---|---|---|
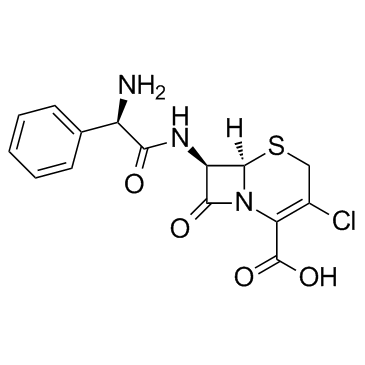 |
cefaclor
CAS:53994-73-3 |
|
 |
5-Methoxy-1H-indole-3-carbaldehyde
CAS:10601-19-1 |
|
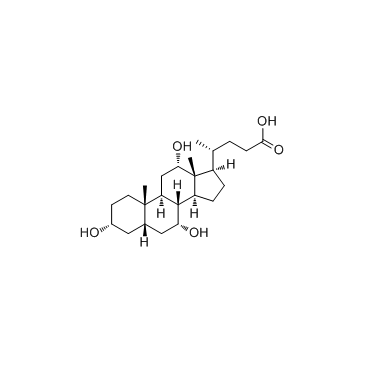 |
cholic acid
CAS:81-25-4 |