5-Methoxy-1H-indole-3-carbaldehyde

5-Methoxy-1H-indole-3-carbaldehyde structure
|
Common Name | 5-Methoxy-1H-indole-3-carbaldehyde | ||
|---|---|---|---|---|
| CAS Number | 10601-19-1 | Molecular Weight | 175.184 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 375.2±22.0 °C at 760 mmHg | |
| Molecular Formula | C10H9NO2 | Melting Point | 179-183 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 180.7±22.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 5-Methoxyindole-3-carboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 375.2±22.0 °C at 760 mmHg |
| Melting Point | 179-183 °C(lit.) |
| Molecular Formula | C10H9NO2 |
| Molecular Weight | 175.184 |
| Flash Point | 180.7±22.3 °C |
| Exact Mass | 175.063324 |
| PSA | 42.09000 |
| LogP | 1.60 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.680 |
| Water Solubility | insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators.
J. Med. Chem. 54 , 5320, (2011) Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance. IDO inhibition is thus an active... |
|
|
Synthesis and antimicrobial activity of cholic acid hydrazone analogues.
Eur. J. Med. Chem. 45 , 2307, (2010) Synthesis and antimicrobial activity of cholic acid analogues 4a-t are reported. The synthesis of 4a-t was accomplished from ethylcholate 2. The hydrazone moiety was introduced via coupling of the cho... |
|
|
Synthesis and in vitro characterization of ionone-based chalcones as novel antiandrogens effective against multiple clinically relevant androgen receptor mutants.
Invest. New Drugs 28 , 291, (2010) A crucial event in prostate cancer progression is the transition from a hormone-sensitive to a lethal castration-refractory disease state. The antagonist-to-agonist conversion due to mutation in AR is... |
| 5-Methoxy-1H-indole-3-carbaldehyde |
| 1H-Indole-3-carboxaldehyde, 5-methoxy- |
| 5-METHOXY-3-INDOLECARBALDEHYDE |
| 5-methoxyindole-3-formaldehyde |
| 3-Formyl-5-methoxyindole |
| 5-Methoxyindole-3-carboxyaldehyde |
| 5-methoxy-1H-indole-3-carboxaIdehyde |
| 5-METHOXYINDOLYL-3-ALDEHYDE |
| 5-methoxyindole-3-carbaldehyde |
| 5-methoxy-1H-indole-3-carboxaldehyde |
| 5-methoxy-1H-indole-3-carbaIdehyde |
| EINECS 234-220-5 |
| 5-METHOXY-3-FORMYLINDOLE |
| 5-METHOXY-3-INDOLECARBOXALDEHYDE |
| MFCD00005623 |
| 5-Methoxyindole-3-carboxaldehyde |
| 5-METHOXYINDOLE-3-ALDEHYDE |
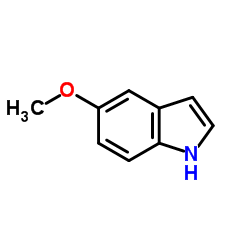 CAS#:1006-94-6
CAS#:1006-94-6 CAS#:68-12-2
CAS#:68-12-2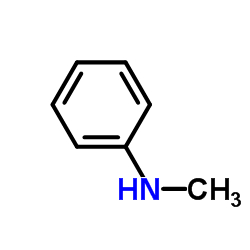 CAS#:100-61-8
CAS#:100-61-8 CAS#:2581-34-2
CAS#:2581-34-2 CAS#:50-00-0
CAS#:50-00-0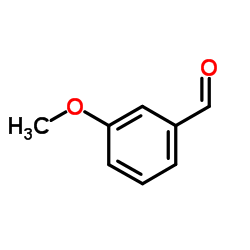 CAS#:591-31-1
CAS#:591-31-1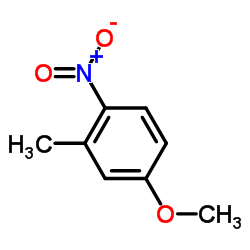 CAS#:5367-32-8
CAS#:5367-32-8 CAS#:77144-90-2
CAS#:77144-90-2 CAS#:39974-94-2
CAS#:39974-94-2 CAS#:608-07-1
CAS#:608-07-1 CAS#:20731-70-8
CAS#:20731-70-8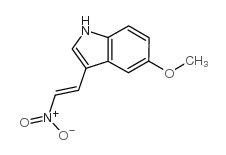 CAS#:61675-19-2
CAS#:61675-19-2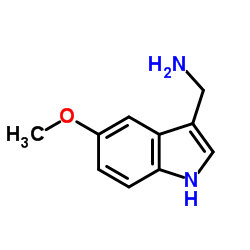 CAS#:60523-82-2
CAS#:60523-82-2 CAS#:154810-58-9
CAS#:154810-58-9 CAS#:145158-71-0
CAS#:145158-71-0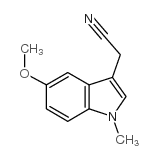 CAS#:176688-98-5
CAS#:176688-98-5 CAS#:191846-76-1
CAS#:191846-76-1
