m-Anisaldehyde
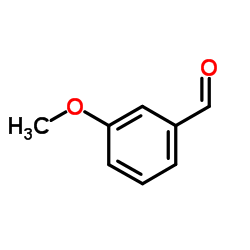
m-Anisaldehyde structure
|
Common Name | m-Anisaldehyde | ||
|---|---|---|---|---|
| CAS Number | 591-31-1 | Molecular Weight | 136.148 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 230.8±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 100.2±13.4 °C | |
Use of m-Anisaldehydem-Anisaldehyde is an endogenous metabolite. |
| Name | 3-Methoxybenzaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | m-Anisaldehyde is an endogenous metabolite. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 230.8±13.0 °C at 760 mmHg |
| Molecular Formula | C8H8O2 |
| Molecular Weight | 136.148 |
| Flash Point | 100.2±13.4 °C |
| Exact Mass | 136.052429 |
| PSA | 26.30000 |
| LogP | 1.65 |
| Vapour Pressure | 0.1±0.5 mmHg at 25°C |
| Index of Refraction | 1.547 |
| InChIKey | WMPDAIZRQDCGFH-UHFFFAOYSA-N |
| SMILES | COc1cccc(C=O)c1 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | BZ2605000 |
| HS Code | 29124900 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2912499000 |
|---|---|
| Summary | 2912499000. other aldehyde-ethers, aldehyde-phenols and aldehydes with other oxygen function. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors.
Bioorg. Med. Chem. 15 , 2006-15, (2007) Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-q... |
|
|
Evidence of13C non-covalent isotope effects obtained by quantitative13C nuclear magnetic resonance spectroscopy at natural abundance during normal phase liquid chromatography
J. Chromatogr. A. 1216(42) , 7043-8, (2009) Quantitative isotopic 13C NMR at natural abundance has been used to determine the site-by-site 13C/ 12C ratios in vanillin and a number of related compounds eluted from silica gel chromatography colum... |
|
|
Interesting anticandidal effects of anisic aldehydes on growth and proton-pumping-ATPase-targeted activity.
Microb. Pathog. 51(4) , 277-84, (2011) Attention has been drawn to evaluate the antifungal activity of p-anisaldehyde (1), o-anisaldehyde (2) and m-anisaldehyde (3). To put forward this approach, antifungal activity has been assessed in th... |
| VHR CO1 |
| 3-Anisaldehyde |
| Metamethoxybenzaldehyde |
| EINECS 209-712-8 |
| 3-Methoxybenzaldehyde |
| 3-Methoxy-benzaldehyde |
| 3-Methoxybezaldehyde |
| m-Methoxybenzaldehyde |
| MFCD00003361 |
| Benzaldehyde, 3-methoxy- |
| 3-monomethoxybenzaldehyde |
| META-ANISALDEHYDE |
| Benzaldehyde,3-methoxy |
| m-Anisaldehyde |
| m-OMe benzaldehyde |
| 3-MeO benzaldehyde |
 CAS#:59184-17-7
CAS#:59184-17-7 CAS#:100-83-4
CAS#:100-83-4 CAS#:74-88-4
CAS#:74-88-4 CAS#:766-85-8
CAS#:766-85-8 CAS#:201230-82-2
CAS#:201230-82-2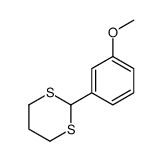 CAS#:54308-43-9
CAS#:54308-43-9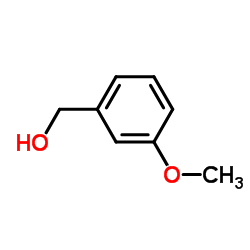 CAS#:6971-51-3
CAS#:6971-51-3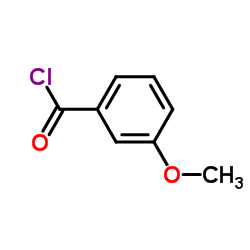 CAS#:1711-05-3
CAS#:1711-05-3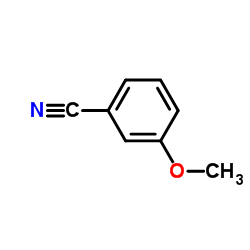 CAS#:1527-89-5
CAS#:1527-89-5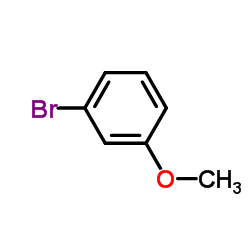 CAS#:2398-37-0
CAS#:2398-37-0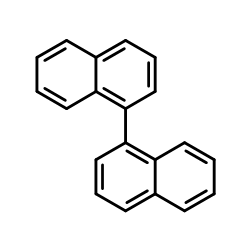 CAS#:604-53-5
CAS#:604-53-5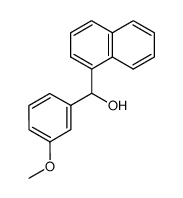 CAS#:6839-11-8
CAS#:6839-11-8 CAS#:109251-88-9
CAS#:109251-88-9 CAS#:109673-21-4
CAS#:109673-21-4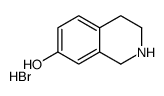 CAS#:110192-19-3
CAS#:110192-19-3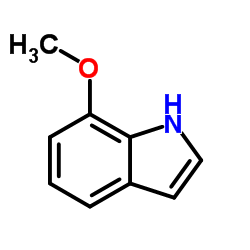 CAS#:3189-22-8
CAS#:3189-22-8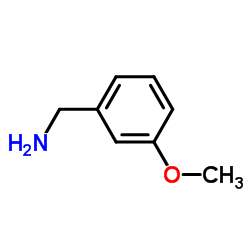 CAS#:5071-96-5
CAS#:5071-96-5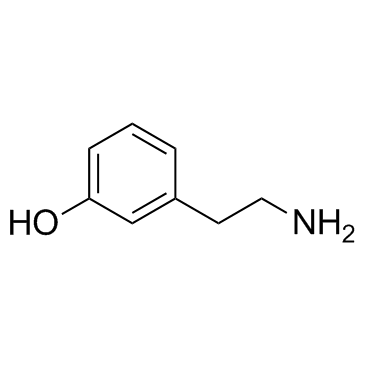 CAS#:588-05-6
CAS#:588-05-6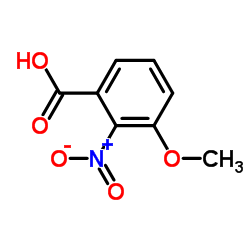 CAS#:4920-80-3
CAS#:4920-80-3 CAS#:42923-77-3
CAS#:42923-77-3
