| Structure | Name/CAS No. | Articles |
|---|---|---|
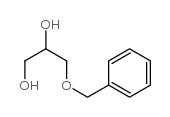 |
3-(Phenylmethoxy)-1,2-propanediol
CAS:4799-67-1 |
|
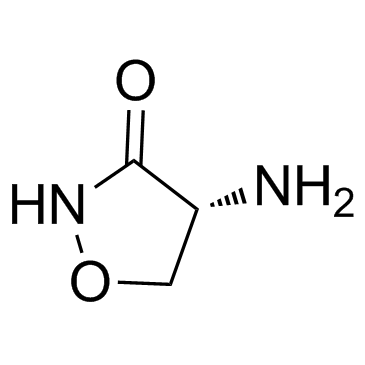 |
D-Cycloserine
CAS:68-41-7 |
|
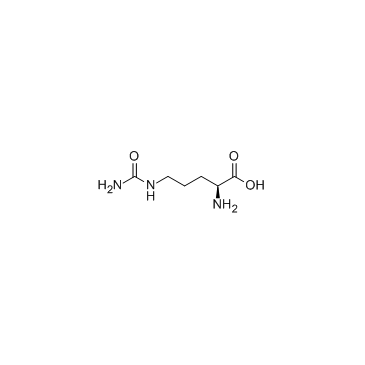 |
L-Citruline
CAS:372-75-8 |
|
 |
Tamoxifen
CAS:10540-29-1 |
|
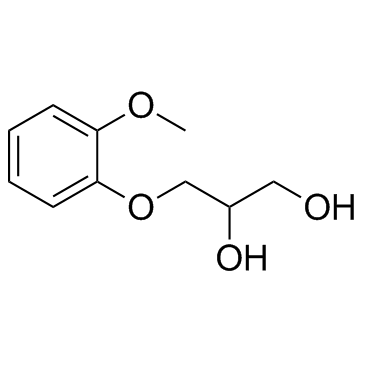 |
Guaifenesin
CAS:93-14-1 |