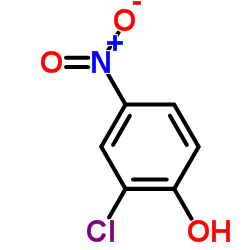
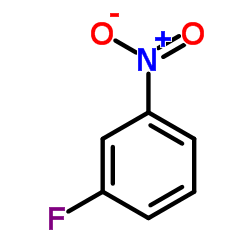
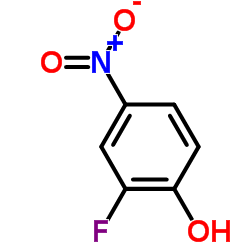
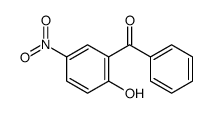
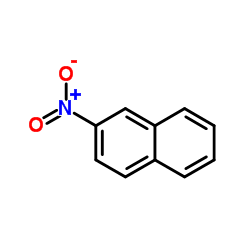
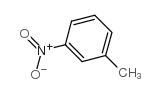
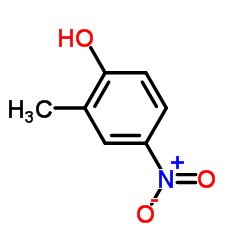
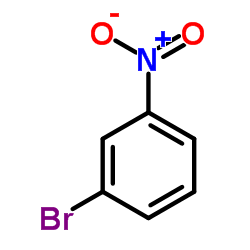
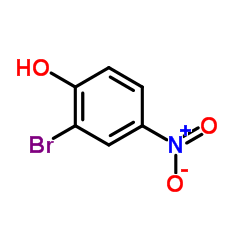
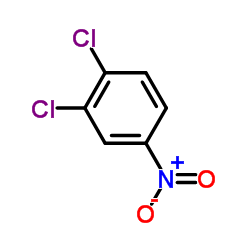
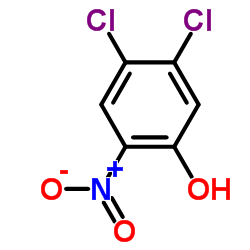
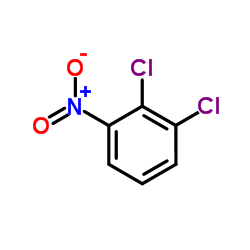
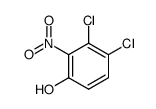
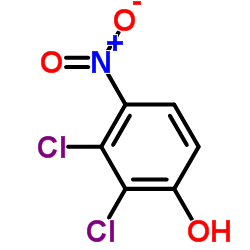
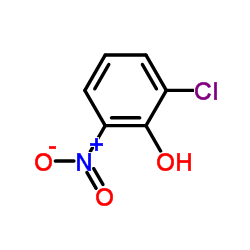
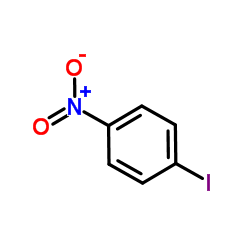
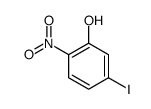
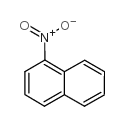
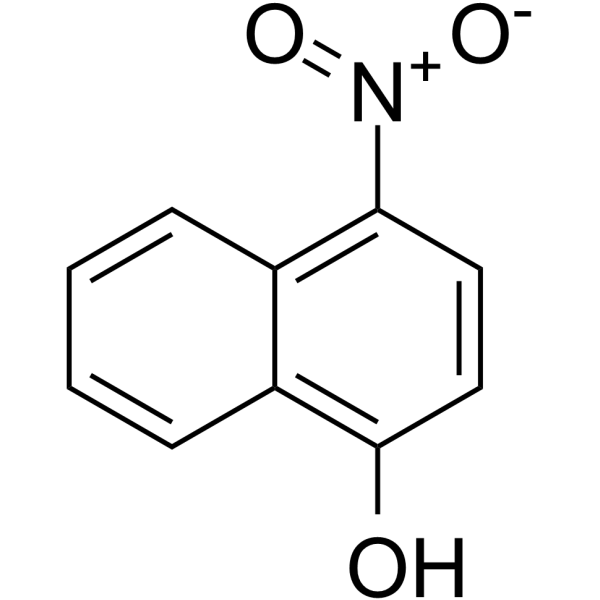
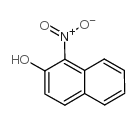
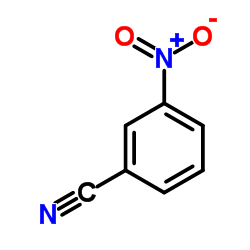
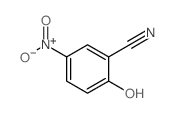
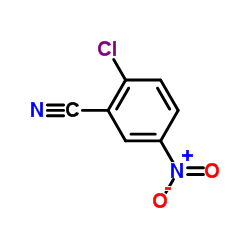
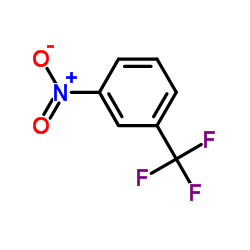
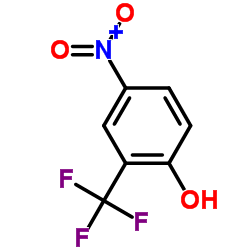
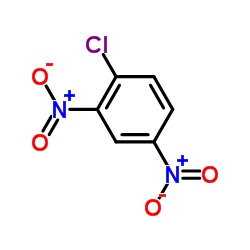
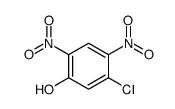
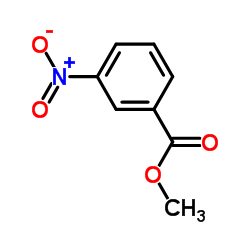
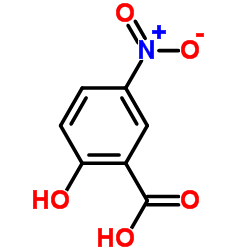
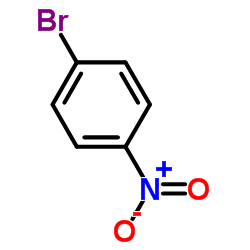
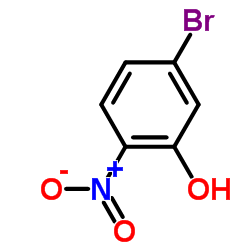
|
~79% |
|
~78% |
|
~76% |
|
~23% |
|
~84% |
|
~18% |
|
~83% |
|
~11% |
|
~66% |
|
~65% |
|
~63% |
|
~86% |
|
~88% |
|
~51% |
|
~78% |
|
~86% |
|
~83% |
|
~89% |
|
~27% |
|
~80% |