| Structure | Name/CAS No. | Articles |
|---|---|---|
![1-Isopropyl-4-methyl-7-oxabicyclo[2.2.1]heptane Structure](https://image.chemsrc.com/caspic/319/470-67-7.png) |
1-Isopropyl-4-methyl-7-oxabicyclo[2.2.1]heptane
CAS:470-67-7 |
|
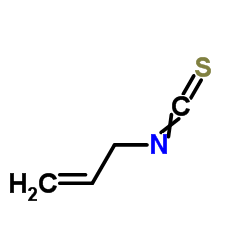 |
Allyl isothiocyanate
CAS:57-06-7 |
|
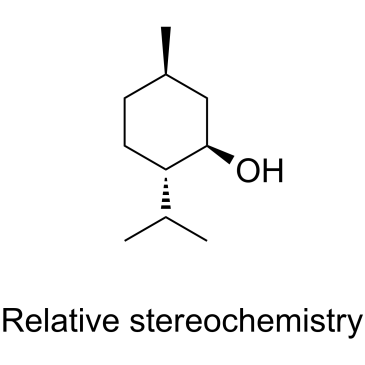 |
Menthol
CAS:89-78-1 |
|
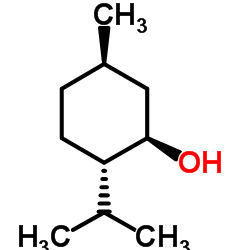 |
Menthol
CAS:1490-04-6 |