| Structure | Name/CAS No. | Articles |
|---|---|---|
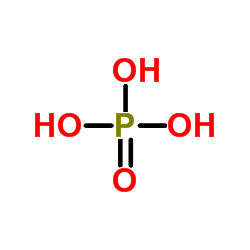 |
Phosphoric acid
CAS:7664-38-2 |
|
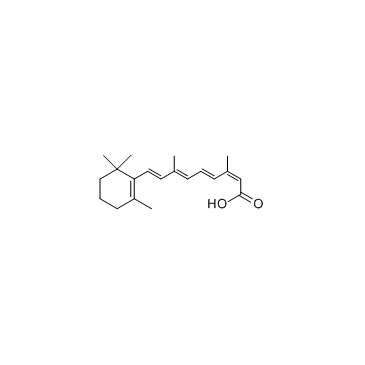 |
Isotretinoin
CAS:4759-48-2 |
|
 |
potassium chloride
CAS:7447-40-7 |
|
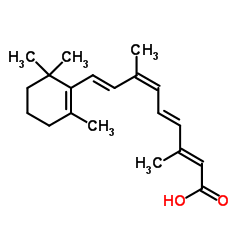 |
Alitretinoin
CAS:5300-03-8 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
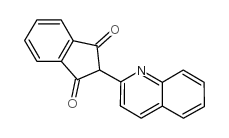 |
Quinoline yellow
CAS:8003-22-3 |
|
 |
Triethylamine
CAS:121-44-8 |
|
 |
Potassium phosphate
CAS:7778-53-2 |