| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
AEBSF HCl
CAS:30827-99-7 |
|
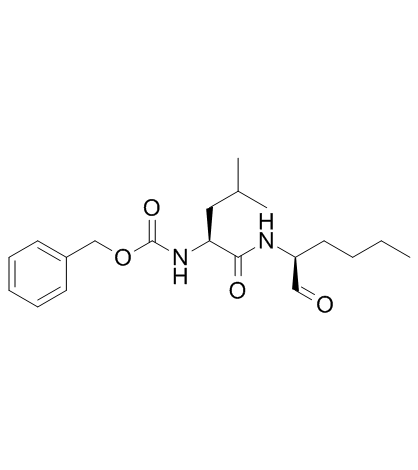 |
calpeptin
CAS:117591-20-5 |
|
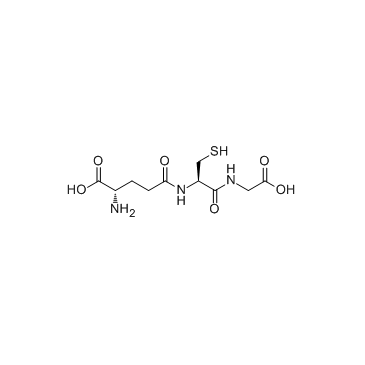 |
Glutathione
CAS:70-18-8 |
|
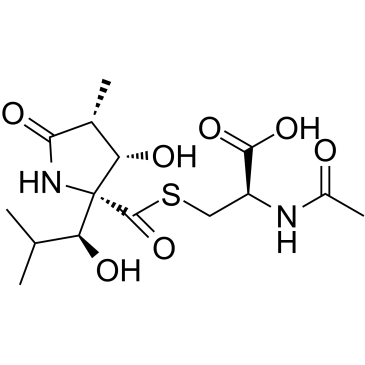 |
Lactacystin
CAS:133343-34-7 |
|
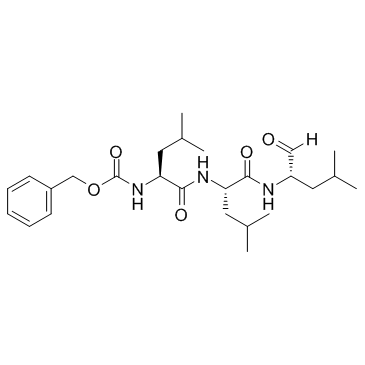 |
MG-132
CAS:133407-82-6 |
|
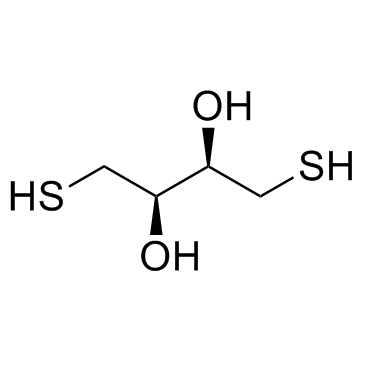 |
DL-Dithiothreitol
CAS:3483-12-3 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
 |
Calpain Inhibitor I
CAS:110044-82-1 |
|
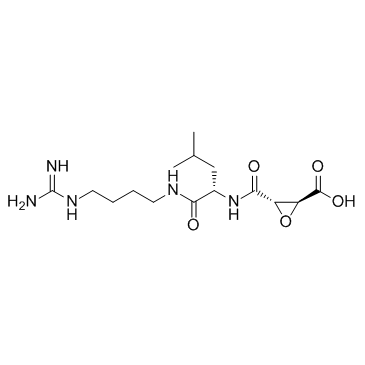 |
E-64
CAS:66701-25-5 |