| Structure | Name/CAS No. | Articles |
|---|---|---|
![Benzo[d]thiazole Structure](https://image.chemsrc.com/caspic/318/95-16-9.png) |
Benzo[d]thiazole
CAS:95-16-9 |
|
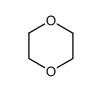 |
1,4-Dioxane
CAS:123-91-1 |
|
 |
Acetone
CAS:67-64-1 |
|
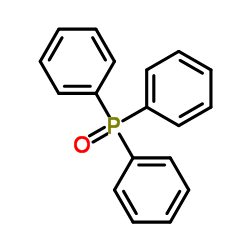 |
Triphenylphosphine oxide
CAS:791-28-6 |
|
 |
Diethyl ether
CAS:60-29-7 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
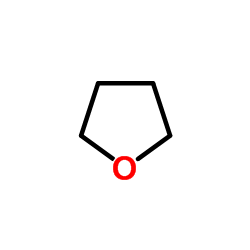 |
thf
CAS:109-99-9 |
|
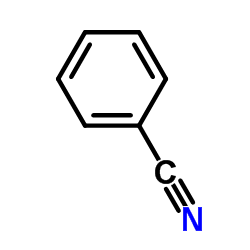 |
Benzonitrile
CAS:100-47-0 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
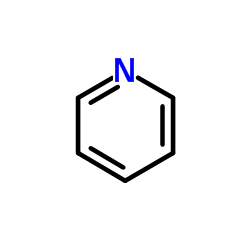 |
Pyridine
CAS:110-86-1 |