| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Aqueous ammonia
CAS:1336-21-6 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
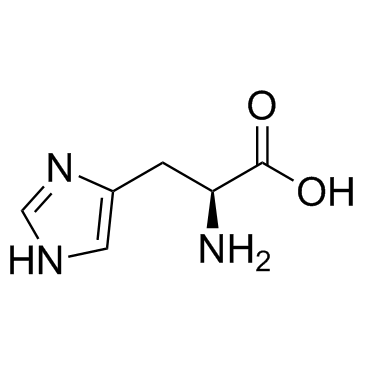 |
L-Histidine
CAS:71-00-1 |
|
 |
Acetyl chloride
CAS:75-36-5 |
|
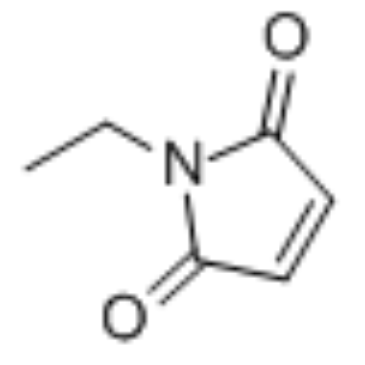 |
N-ethylmaleimide
CAS:128-53-0 |
|
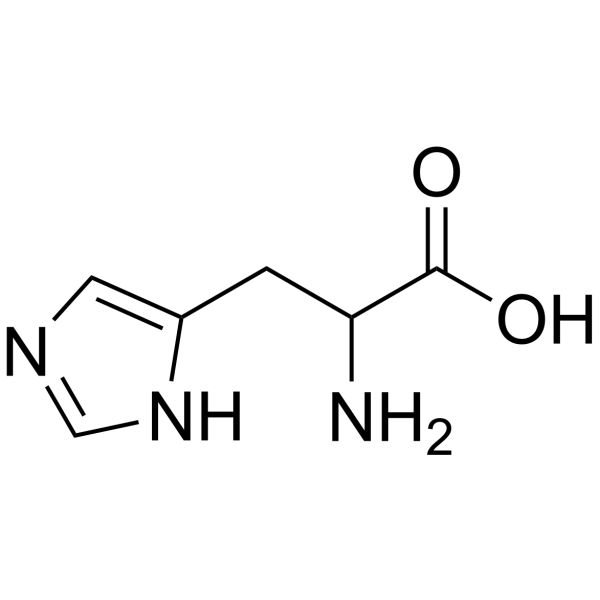 |
DL-Histidine
CAS:4998-57-6 |
|
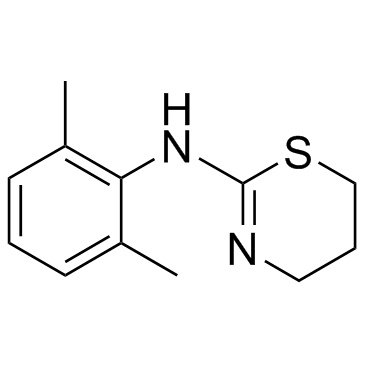 |
Xylazine
CAS:7361-61-7 |