| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodiumborohydride
CAS:16940-66-2 |
|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
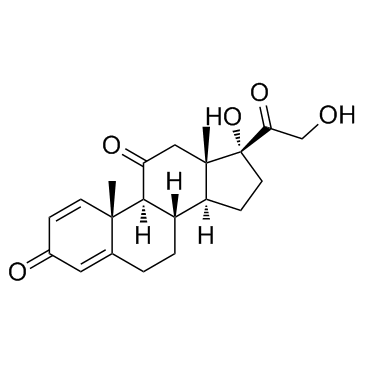 |
Prednisone
CAS:53-03-2 |
|
 |
Methanol
CAS:67-56-1 |
|
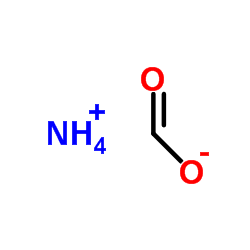 |
Formic acid ammonium salt
CAS:540-69-2 |
|
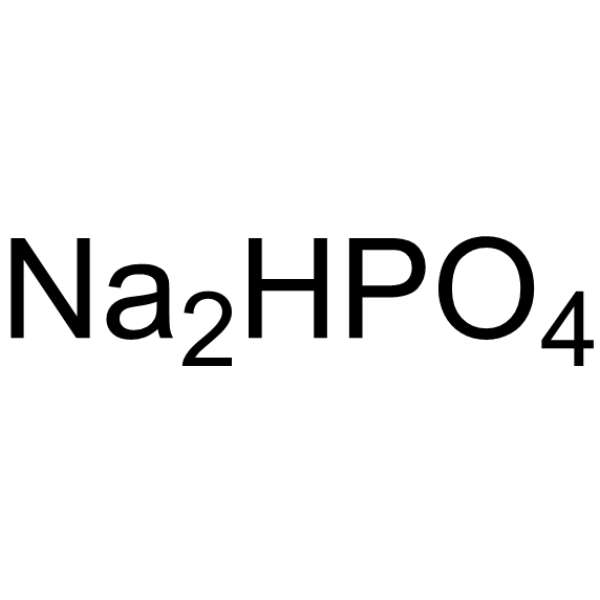 |
Disodium hydrogenorthophosphate
CAS:7558-79-4 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
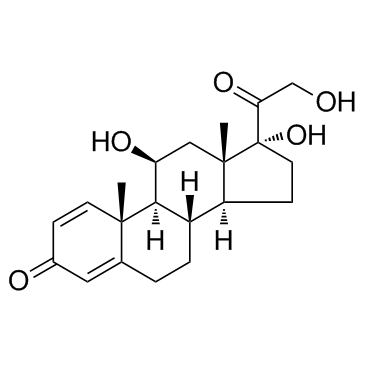 |
prednisolone
CAS:50-24-8 |
|
 |
Ammonium Chloride
CAS:12125-02-9 |