| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Formic Acid
CAS:64-18-6 |
|
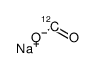 |
sodium,oxomethanolate
CAS:1218765-26-4 |
|
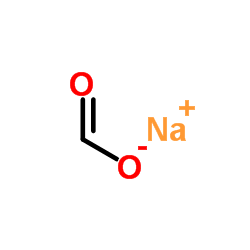 |
Sodium formate
CAS:141-53-7 |
|
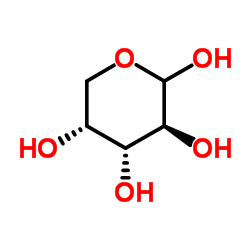 |
D-Arabinose
CAS:10323-20-3 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
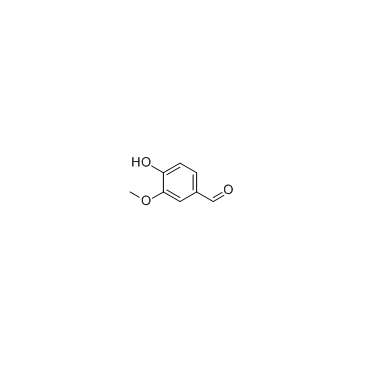 |
Vanillin
CAS:121-33-5 |
|
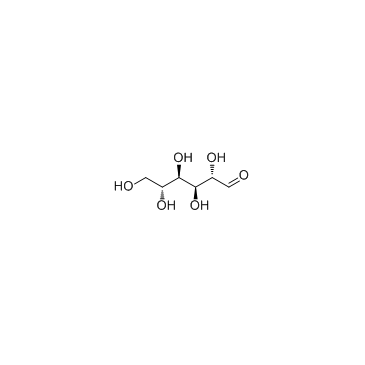 |
D-Mannose
CAS:3458-28-4 |
|
 |
acetic acid
CAS:64-19-7 |