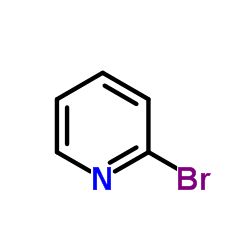
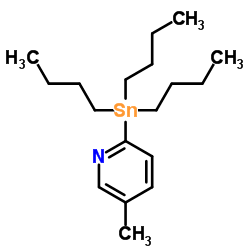
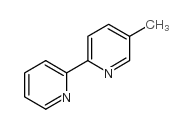
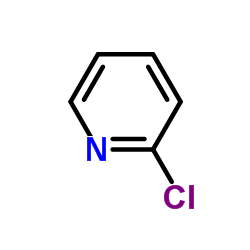
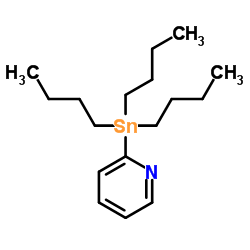
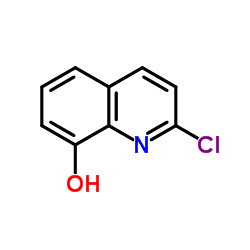
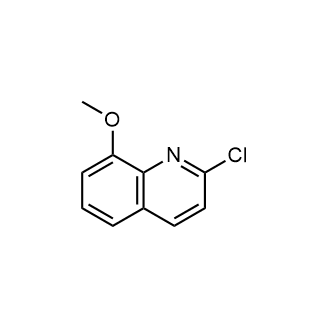
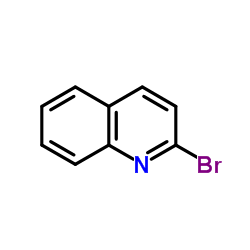
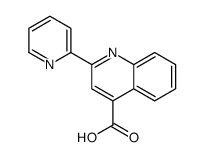
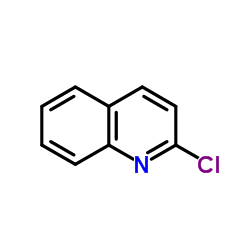
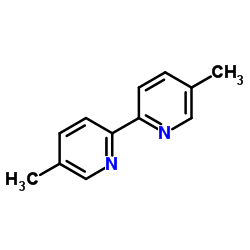
|
~64% |
|
~43% |
|
~40% |
|
~97% |
|
~75% |
|
~57% |
|
~69% |
|
~51% |
|
~35% |