Proscillaridin A
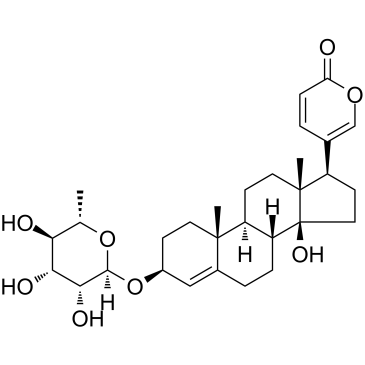
Proscillaridin A structure
|
Common Name | Proscillaridin A | ||
|---|---|---|---|---|
| CAS Number | 466-06-8 | Molecular Weight | 530.650 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 716.7±60.0 °C at 760 mmHg | |
| Molecular Formula | C30H42O8 | Melting Point | 233ºC | |
| MSDS | Chinese USA | Flash Point | 232.6±26.4 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Pulse pressure correlates in humans with a proscillaridin A immunoreactive compound.
Hypertension 27(5) , 1073-8, (1996) Endogenous digitalis-like factors in humans are presumably cardenolides and bufadienolides. To test whether bufadienolide-like substances may circulate in human blood, we used antibodies from rabbits against the bufadienolide proscillaridin A to measure the c... |
|
|
Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs.
J. Nat. Prod. 72 , 1969-74, (2009) Cardiac glycosides have been reported to exhibit cytotoxic activity against several different cancer types, but studies against colorectal cancer are lacking. In a screening procedure aimed at identifying natural products with activity against colon cancer, s... |
|
|
Role of endogenous cardiac glycosides in the spontaneously hypertensive rat--antagonism by active immunization.
Am. J. Hypertens. 9(1) , 81-5, (1996) The effects of simultaneous active immunization against two cardiac glycoside drugs, digoxin and proscillaridin, have been examined in young spontaneously hypertensive and Wistar-Kyoto rats. Control animals were immunized with protein carrier only. Animals we... |
|
|
Specific anti-digoxin Fab fragments: an available antidote for proscillaridin and scilliroside poisoning?
Hum. Exp. Toxicol. 9(3) , 191-3, (1990) The purpose of this study was to evaluate the capacity of specific anti-digoxin Fab fragments to bind to and neutralize scilliroside and proscillaridin in acute poisoning. Apparent affinity constants were determined with values of 2.6 10(8)M-1 for scillirosid... |
|
|
Studies on cardiac ingredients of plants. IX. Chemical transformation of proscillaridin by utilizing its 1,4-cycloadducts as key compounds and biological activities of their derivatives.
Chem. Pharm. Bull. 40(2) , 327-32, (1992) Three aromatic compounds (2-4) possessing a carbomethoxyl group or a dimethoxyphthaloyl group, prepared by the Diels-Alder reaction of the cardiac glycoside, proscillaridin (1), with dimethyl acetylenedicarboxylate and methyl propiolate, were transformed into... |
|
|
Proscillaridin A immunoreactivity: its purification, transport in blood by a specific binding protein and its correlation with blood pressure.
Clin. Exp. Hypertens. 20(5-6) , 593-9, (1998) A material crossreacting with antibodies against the bufadienolide proscillaridin A and inhibiting the sodium pump was found in human blood plasma. The concentration of the material with a retention time similar to ouabain in a reversed phase HPLC correlated ... |
|
|
Cardiac glycoside-induced elevation of intracellular Na+ ion concentration in human erythrocytes studied by 23Na NMR spectroscopy: relationship between inotropy speed and elevation rate of intracellular Na+ ion concentration.
Biol. Pharm. Bull. 16(4) , 431-3, (1993) Elevation of intracellular sodium ion concentration in human erythrocyte induced by the cardiac glycoside, proscillaridin, and its four derivatives was measured using 23Na NMR spectrometry. In this examination, there was a significant correlation between the ... |
|
|
The bioavailability of methylepoxyproscillaridin (P35): a new semisynthetic cardiac glycoside.
Int. J. Clin. Pharmacol. Ther. Toxicol. 26(6) , 297-9, (1988) The pharmacokinetic data of methylepoxyproscillaridin (3'-methyl-4'-5'-epoxyproscillaridin) (P35) after 1 mg i.v. or oral administration in two different galenic preparations (hard and soft gelatin capsules) in randomized succession were studied in 9 healthy ... |
|
|
[Studies on cardiac ingredients of plants. VIII. Preparation of nitrates of proscillaridin and their pharmacological activities].
Yakugaku Zasshi 111(8) , 436-44, (1991) To reduce the vascular contracting effect of the cardiac glycoside, proscillaridin (1), all kinds of its nitrates were prepared by utilizing effectively an isopropylidene function as a protective group. The pharmacological activities of proscillaridin nitrate... |
|
|
Inhibition of DNA topoisomerases I and II by G3 PAMAM-NH2 dendrimer-modified digoxin and proscillaridin A conjugates in a cell free system.
Acta Pol. Pharm. 67(6) , 630-4, (2010) Two modified glycosides--digoxin and proscillaridin A conjugated to a generation 3 of polyamidoamine dendrimer (G3 PAMAM-NH2) were evaluated as DNA topoisomerase II inhibitors. The ability of these compounds (PAMAM-Dig and PAMAM-Prosc) to inhibit topoisomeras... |