Magnesium oxide
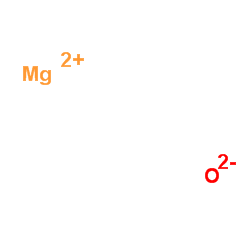
Magnesium oxide structure
|
Common Name | Magnesium oxide | ||
|---|---|---|---|---|
| CAS Number | 1309-48-4 | Molecular Weight | 40.304 | |
| Density | 3.58 | Boiling Point | 3600ºC | |
| Molecular Formula | MgO | Melting Point | 2852ºC | |
| MSDS | Chinese USA | Flash Point | 3600ºC | |
|
Modulation of microenvironmental pH and utilization of alkalizers in crystalline solid dispersion for enhanced solubility and stability of clarithromicin.
Arch. Pharm. Res. 38 , 839-48, (2015) Clarithromycin (CAM) is known to be poorly water-soluble and acid-labile drug. Various alkalizers such as MgO, Na2CO3, Na2HPO4 and NaHCO3 were utilized to modulate the microenvironmental pH (pHM) and to improve the low stability and solubility of CAM in a cry... |
|
|
Hydrogenation of quinolines, alkenes, and biodiesel by palladium nanoparticles supported on magnesium oxide.
Dalton Trans. 41(48) , 14490-7, (2012) A new catalyst composed of Pd nanoparticles supported on MgO has been prepared by the room temperature NaBH(4) reduction of Na(2)PdCl(4) in methanol in the presence of the support. TEM measurements reveal well-dispersed Pd particles of mean diameter 1.7 nm at... |
|
|
Effect of partial crystallization on the mechanical properties and cytotoxicity of bioactive glass from the 3CaO.P(2)O(5)-SiO(2)-MgO system.
J. Mech. Behav. Biomed. Mater. 14 , 78-88, (2012) The aim of this study is to report on the development and characterization of bioactive glass and glass-ceramics from the 3CaO.P(2)O(5)-SiO(2)-MgO-system, using different degrees of cristallinity for applications as an implant material. A methodology was prop... |
|
|
Evaluation of enantiomeric purity of magnesium-L-aspartate dihydrate.
J. Pharm. Biomed. Anal. 102 , 100-9, (2014) Magnesium supplementation in form of organic magnesium salts is a very popular practice. We examined the enantiomeric purity of "Magnesium aspartate dihydrate" monographed in the European Pharmacopeia. A chiral capillary zone electrophoresis using (2-hydroxyp... |
|
|
Droplet sampling of an oil-based and two water-based antievaporant ultra-low volume insecticide formulations using Teflon- and magnesium oxide-coated slides.
J. Am. Mosq. Control Assoc. 29(2) , 173-6, (2013) We estimated the diameters below which 50% and 90% of the volume of droplets exist (Dv50 and Dv90, respectively) of 1 oil-based (Permanone 30-30) and 2 water-based (AquaReslin, Aqua-K-Othrine) antievaporant aerosols (with the Film Forming Aqueous Spray Techno... |
|
|
Solid state compatibility study and characterization of a novel degradation product of tacrolimus in formulation.
J. Pharm. Biomed. Anal. 110 , 67-75, (2015) Tacrolimus is macrolide drug that is widely used as a potent immunosuppressant. In the present work compatibility testing was conducted on physical mixtures of tacrolimus with excipients and on compatibility mixtures prepared by the simulation of manufacturin... |
|
|
Decrease in ciprofloxacin absorption by polyvalent metal cations is not fully attributable to chelation or adsorption.
Drug Metab. Pharmacokinet. 29(5) , 414-8, (2014) The drug interaction between new quinolone antibiotics (NQs) and polyvalent metal cation products, leading to a significant decrease in the absorption of NQ, is considered to be attributable to the formation of poorly absorbable chelate and physicochemical ad... |
|
|
The effects of screw configuration and polymeric carriers on hot-melt extruded taste-masked formulations incorporated into orally disintegrating tablets.
J. Pharm. Sci. 104(1) , 124-34, (2014) The primary aim of this research was to produce successfully taste masked formulations of Sildenafil Citrate (SC) using hot-melt extrusion (HME) technology. Multiple screw configurations and polymeric carriers were evaluated for their effects on taste masking... |
|
|
Stabilisation of amorphous ibuprofen in Upsalite, a mesoporous magnesium carbonate, as an approach to increasing the aqueous solubility of poorly soluble drugs.
Int. J. Pharm. 472(1-2) , 185-91, (2014) One attractive approach to increase the aqueous solubility and thus the bioavailability of poorly soluble drugs is to formulate them in their amorphous state since amorphous compounds generally exhibit higher apparent solubilities than their crystalline count... |
|
|
Isotope concentrations from 24-h urine and 3-h serum samples can be used to measure intestinal magnesium absorption in postmenopausal women.
J. Nutr. 144(4) , 533-7, (2014) Studies suggest a link between magnesium status and osteoporosis. One barrier to more conclusive research on the potential relation is measuring intestinal magnesium absorption (MgA), which requires the use of stable isotopes and a ≥6-d stool or 3-d urine col... |