| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
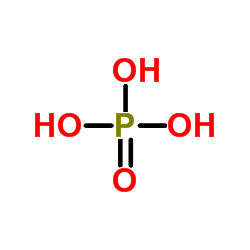 |
Phosphoric acid
CAS:7664-38-2 |
|
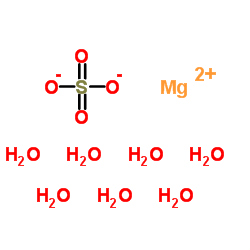 |
magnesium sulfate heptahydrate
CAS:10034-99-8 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
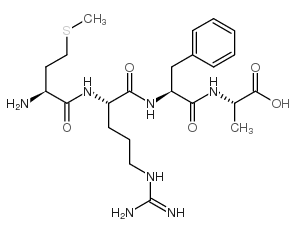 |
H-Met-Arg-Phe-Ala-OH
CAS:67368-29-0 |
|
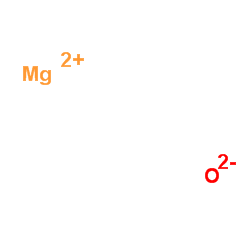 |
Magnesium oxide
CAS:1309-48-4 |
|
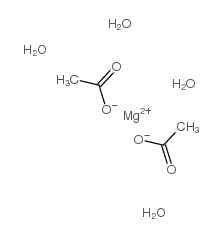 |
Magnesium acetate tetrahydrate
CAS:16674-78-5 |
|
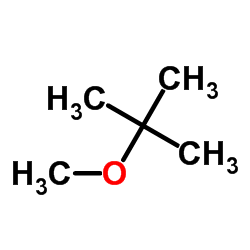 |
Methyl tert-butyl ether
CAS:1634-04-4 |