Methyl (R)-(-)-3-hydroxybutyrate
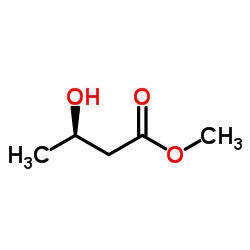
Methyl (R)-(-)-3-hydroxybutyrate structure
|
Common Name | Methyl (R)-(-)-3-hydroxybutyrate | ||
|---|---|---|---|---|
| CAS Number | 3976-69-0 | Molecular Weight | 118.131 | |
| Density | 1.055 | Boiling Point | 161.9±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H10O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 71.7±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
A novel carbonyl reductase with anti-Prelog stereospecificity from Acetobacter sp. CCTCC M209061: purification and characterization.
PLoS ONE 9(4) , e94543, (2014) A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The en... |
|
|
First total synthesis of grahamimycin A.
J. Org. Chem. 65 , 7221, (2000)
|
|
|
Efficient asymmetric synthesis of radicicol dimethyl ether: a novel application of ring-forming olefin metathesis.
Org. Lett. 2 , 3127, (2000) A concise, stereospecific synthesis of radicicol dimethyl ether is presented. The strategy relies on a convergent three-stage assembly of the 14-membered lactone which has, as a key transformation, a novel ring-forming metathesis reaction utilizing a vinyl ep... |
|
|
A new synthesis of a key intermediate of beta-lactam antibiotics via diastereoselective alkylation of beta-hydroxy ester.
J. Org. Chem. 65 , 8372, (2000)
|
|
|
A. Fischli, R. Scheffold, ed.
Modern Synthetic Methods Salle+Sauerländer 2 , 269, (1980)
|