Clobetasol
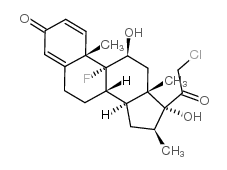
Clobetasol structure
|
Common Name | Clobetasol | ||
|---|---|---|---|---|
| CAS Number | 25122-41-2 | Molecular Weight | 410.90700 | |
| Density | 1.32 g/cm3 | Boiling Point | 555.1ºC at 760 mmHg | |
| Molecular Formula | C22H28ClFO4 | Melting Point | 195 - 197C (decomposes)ºC | |
| MSDS | Chinese USA | Flash Point | 289.5ºC | |
| Symbol |

GHS08 |
Signal Word | Danger | |
|
Topical steroids for chronic wounds displaying abnormal inflammation.
Ann. R. Coll. Surg. Engl. 95(4) , 291-6, (2013) Chronic, non-healing wounds are often characterised by an excessive, and detrimental, inflammatory response. We review our experience of using a combined topical steroid, antibiotic and antifungal preparation in the treatment of chronic wounds displaying abno... |
|
|
Impact of clobetasol propionate 0.05% spray on health-related quality of life in patients with plaque psoriasis.
Journal. of. Drugs in. Dermatology. 11(11) , 1348-54, (2012) Psoriasis causes significant distress and impairment in health-related quality of life (QOL) in afflicted patients. For this reason, QOL is an essential and important measure of treatment outcome in patients with the disease. Clobetasol propionate is a super-... |
|
|
Clobetasol propionate spray 0.05% for the treatment of moderate to severe plaque psoriasis.
Cutis. 89(2) , 89-94, (2012) Clobetasol propionate is a super-high potent class 1 topical corticosteroid available in several formulations, including a spray formulation that is approved for use up to 4 weeks in patients aged 18 years and older with moderate to severe plaque psoriasis. T... |
|
|
Influence of the type of vegetable oil on the drug release profile from lipid-core nanocapsules and in vivo genotoxicity study.
Pharm. Dev. Technol. 19(7) , 789-98, (2014) The use of rice bran (RB), soybean (SB) or sunflower seed (SF) oils to prepare lipid-core nanocapsules (LNCs) as controlled drug delivery systems was investigated. LNCs were prepared by interfacial deposition using the preformed polymer method. All formulatio... |
|
|
Beefy red plaques in the perianal region.
JAMA Dermatol. 150(4) , 447-8, (2014)
|
|
|
A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus.
J. Am. Acad. Dermatol. 71(1) , 84-91, (2014) Vulvar lichen sclerosus is a chronic condition usually responsive to topical corticosteroids.We sought to evaluate the efficacy (reduction of signs and symptoms) and safety of clobetasol propionate 0.05% and tacrolimus 0.1% in the treatment of vulvar lichen s... |
|
|
Clobetasol propionate for psoriasis: are ointments really more potent?
Journal. of. Drugs in. Dermatology. 5(6) , 527-32, (2006) Clobetasol propionate is the most common topical therapy used for psoriasis in the US. Conventional dermatologic wisdom is that ointment preparations provide the highest potency (due to their occlusive nature and moisturizing ability) and are best suited for ... |
|
|
Clobetasol propionate foam, 0.05%.
Am. J. Clin. Dermatol. 2(2) , 89-92; discussion 93, (2001) Clobetasol propionate foam (clobetasol foam), a new formulation of the superpotent corticosteroid, has anti-inflammatory, antipruritic and vasoconstrictive properties. It was reported that the absorption rate of clobetasol was greater from the foam than from ... |
|
|
Topical clobetasol propionate in the treatment of psoriasis: a review of newer formulations.
Am. J. Clin. Dermatol. 10(6) , 397-406, (2009) Ultrapotent topical corticosteroids are the mainstay of psoriasis treatment, used either alone or in combination with a topical vitamin D analog. Traditionally used in an ointment vehicle for psoriasis, clobetasol propionate 0.05% is also available in spray, ... |
|
|
Iatrogenic Cushing's syndrome and topical steroid therapy: case series and review of the literature.
J. Dermatolog. Treat. 25(6) , 495-500, (2014) Topical corticosteroids are considered first-line therapy in patients with chronic inflammatory oral mucosal diseases; among them, clobetasol propionate is one of the most widely used in oral medicine. Under physiological conditions, the transmucosal applicat... |