1-(Furan-2-yl)ethanone
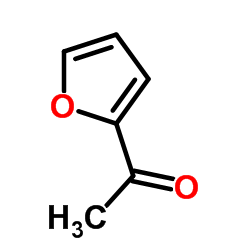
1-(Furan-2-yl)ethanone structure
|
Common Name | 1-(Furan-2-yl)ethanone | ||
|---|---|---|---|---|
| CAS Number | 1192-62-7 | Molecular Weight | 110.111 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 183.4±0.0 °C at 760 mmHg | |
| Molecular Formula | C6H6O2 | Melting Point | 26-28 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 71.1±0.0 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
|
Quantification of furanic derivatives in fortified wines by a highly sensitive and ultrafast analytical strategy based on digitally controlled microextraction by packed sorbent combined with ultrahigh pressure liquid chromatography.
J. Chromatogr. A. 1381 , 54-63, (2015) An improved, reliable and powerful analytical strategy based on digitally controlled microextraction by packed sorbent (MEPS) combined with ultrahigh pressure liquid chromatography (UHPLC) was validated for the simultaneous identification and quantification o... |
|
|
Dose-dependent increase in 2,5-hexanedione in the urine of workers exposed to n-hexane.
Int. Arch. Occup. Environ. Health 63(4) , 285-91, (1991) The concentrations of 2,5-hexanedione (2,5-HD), an n-hexane metabolite, and 2-acetylfuran (2-AF) were measured in urine samples from 123 workers who had predominantly been exposed to n-hexane vapor and 53 workers who had experienced no exposure to solvents. T... |
|
|
2-Acetylfuran, a confounder in urinalysis for 2,5-hexanedione as an n-hexane exposure indicator.
Int. Arch. Occup. Environ. Health 63(3) , 213-9, (1991) The apparent amount of 2,5-hexanedione, a biomarker of n-hexane expsoure in occupational health, in the urine of both exposed and non-exposed subjects varied not only as a function of the pH at which the urine sample was hydrolyzed but also depending on the c... |
|
|
[Acute hepatitis in subjects exposed to 2-acetylfuran and hydrazine].
Med. Lav. 74(4) , 284-90, (1983)
|
|
|
Spectral studies on Co(II), Ni(II) and Cu(II) complexes with thiosemicarbazone (L1) and semicarbazone (L2) derived from 2-acetyl furan.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(4-5) , 1347-51, (2007) Co(II), Ni(II) and Cu(II) complexes are synthesized with thiosemicarbazone (L1) and semicarbazone (L2) derived from 2-acetyl furan. These complexes are characterized by elemental analysis, molar conductance, magnetic susceptibility measurements, mass, IR, ele... |
|
|
Comparison of 2-acetylfuran formation between ribose and glucose in the Maillard reaction.
J. Agric. Food Chem. 56(24) , 11997-2001, (2008) Sugar type is a major factor regulating the reaction rates and pathways in Maillard reaction. Ribose and glucose were used to compare their reactivities and pathways of 2-acetylfuran formation. A stable isotope labeling method was used to study their reactivi... |
|
|
An evaluation of the antirhinoviral activity of acetylfuran replacements for 3-methylisoxazoles. Are 2-acetylfurans bioisosteres for 3-methylisoxazoles?
J. Med. Chem. 37(24) , 4177-84, (1994) As a probe of the 3-methylisoxazole portion of our broad-spectrum antipicornaviral series, a panel of 2-acetylfuran analogues was prepared as replacements for the 3-methylisoxazole ring. Comparison of the two series showed remarkable similarity in potency, sp... |
|
|
Short and efficient synthetic route to methyl α-trioxacarcinoside B and anomerically activated derivatives.
Org. Lett. 13(20) , 5584-7, (2011) A 9-step synthetic route to the complex carbohydrate methyl α-trioxacarcinoside B from 2-acetylfuran is described. Anomerically activated forms, including 1-phenylthio, 1-O-(4'-pentenyl), 1-fluoro, and 1-O-acetyl derivatives are also prepared.© 2011 American ... |
|
|
Organoleptic Characteristics of Flavor Materials Mosciano, G.
Perfum. Flavor. 6th ed., 28 , 76, (2003)
|