5-Methyl-2-furaldehyde
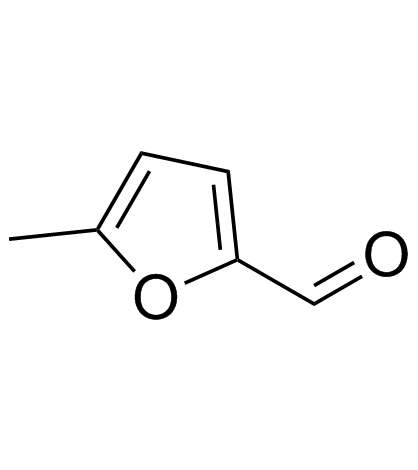
5-Methyl-2-furaldehyde structure
|
Common Name | 5-Methyl-2-furaldehyde | ||
|---|---|---|---|---|
| CAS Number | 620-02-0 | Molecular Weight | 110.111 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 187.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C6H6O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 72.8±0.0 °C | |
|
Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: phenolic, volatile, and mineral compounds.
J. Agric. Food Chem. 62(45) , 10861-72, (2014) Vine-shoots are an important waste in all viticulture areas that should be re-used with innovative applications. The aim of this work was to produce Airén waste vine-shoot aqueous extracts by four solid-liquid extraction techniques such as conventional solid-... |
|
|
Effect of adulteration versus storage on volatiles in unifloral honeys from different floral sources and locations.
J. Food Sci. 78(2) , C184-91, (2013) High fructose corn syrup (HFCS) was added at 5% to 40% to Indiana wildflower honey and added at 40% to Ohio and Indiana honeys from blueberry, star thistle, clover and wildflower, and an unknown source to simulate honey adulteration. Unadulterated honeys were... |
|
|
Specificity of the in vitro interaction of methylfurfural with DNA.
Mutagenesis 5(2) , 131-6, (1990) Methylfurfural (MF) or 5-methyl 2-furaldehyde is a dietary mutagen and is present in various food products and beverages. Alkaline unwinding of calf thymus DNA and the protection of cleavage sites in lambda phage DNA from the action of various restriction enz... |
|
|
Simultaneous determination of 2-furfural and 5-methyl-2-furfural using corona discharge ion mobility spectrometry.
Anal. Sci. 25(6) , 801-5, (2009) A novel technique, corona discharge ion mobility spectrometry (CD-IMS), was developed for the qualitative and quantitative determination of 2-furfural (F) and 5-methyl-2-furfural (MF) in aqueous solutions. The limits of detection (LODs) were 5.3 x 10(-3) micr... |
|
|
Metabolism of 5-methyl-2-furaldehyde in rat. III. Identification and determination of 5-methyl-2-furylmethylketone.
Xenobiotica 18(8) , 887-92, (1988) 1. Continuous extraction, column chromatography and t.l.c. were employed to isolate a minor metabolite of 5-methyl-2-furaldehyde from rat urine. 2. The metabolite was identified by mass spectrometry and independent synthesis as 5-methyl-2-furylmethylketone. 3... |
|
|
Formation of furan and methylfuran by maillard-type reactions in model systems and food.
J. Agric. Food Chem. 56(10) , 3639-47, (2008) The formation of furan and 2-methylfuran was studied in model systems based on sugars and selected amino acids. Both compounds were preferably formed under roasting conditions in closed systems yielding up to 330 micromol of furan and 260 micromol of 2-methyl... |
|
|
Isolation and characterization of Cupriavidus basilensis HMF14 for biological removal of inhibitors from lignocellulosic hydrolysate.
Microb. Biotechnol. 3(3) , 336-43, (2010) The formation of toxic fermentation inhibitors such as furfural and 5-hydroxy-2-methylfurfural (HMF) during acid (pre-)treatment of lignocellulose, calls for the efficient removal of these compounds. Lignocellulosic hydrolysates can be efficiently detoxified ... |
|
|
[Gas-chromatographic determination of furfural, methylfurfural and furyl alcohol in air].
Gig. Tr. Prof. Zabol. (12) , 53-4, (1985)
|
|
|
Synthesis, structure and biological activity of nickel(II) complexes of 5-methyl 2-furfural thiosemicarbazone.
J. Inorg. Biochem. 86(2-3) , 565-71, (2001) 5-Methyl 2-furfuraldehyde thiosemicarbazone (M5HFTSC) with nickel(II) leads to three types of complexes: [Ni(M5HFTSC)(2)X(2)], [Ni(M5FTSC)(2)] and [Ni(M5FTSC)(2)] x 2DMF. In the first type the ligand remains in thione form, while in the two other, the anionic... |
|
|
Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry using isotope dilution.
J. Chromatogr. A. 1017(1-2) , 141-9, (2003) A new method was developed for the determination of 2-furfural (2-F) and 5-methylfurfural (5-MF), two products of Maillard reaction in vinegar, with head-space solid-phase microextraction (HS-SPME) coupled to gas chromatography-mass spectrometry (GC-MS). A di... |