(-)-Diethyl D-tartrate
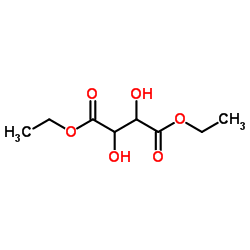
(-)-Diethyl D-tartrate structure
|
Common Name | (-)-Diethyl D-tartrate | ||
|---|---|---|---|---|
| CAS Number | 13811-71-7 | Molecular Weight | 206.193 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 280.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H14O6 | Melting Point | 17ºC | |
| MSDS | USA | Flash Point | 93.3±0.0 °C | |
|
Bioplastics from feather quill.
Biomacromolecules 12(10) , 3826-32, (2011) Poultry feather quills have been extruded in a twin screw extruder with sodium sulfite treatment as a reducing agent. The effect of four different plasticizers (ethylene glycol, propylene glycol, glycerol, and diethyl tartrate) on the thermoplastic properties... |
|
|
Separation of corticosteroids by microemulsion EKC with diethyl L-tartrate as the oil phase.
Electrophoresis 28(20) , 3691-6, (2007) A novel microemulsion based on a mixture of diethyl L-tartrate (DET) and SDS was developed for the microemulsion EKC (MEEKC) determination of structurally related steroids. The system consisted of 0.5% w/w DET, 1.7% w/w SDS, 1.2% w/w 1-butanol, 89.6% w/w phos... |
|
|
A practical and azide-free synthetic approach to oseltamivir from diethyl D-tartrate.
J. Org. Chem. 75(9) , 3125-8, (2010) A short and practical synthesis of oseltamivir was accomplished in 11 steps from inexpensive and abundant diethyl D-tartrate starting material. This azide-free route featured an asymmetric aza-Henry reaction and a domino nitro-Michael/Horner-Wadsworth-Emmons ... |
|
|
Two-chiral component microemulsion EKC - chiral surfactant and chiral oil. Part 2: diethyl tartrate.
Electrophoresis 28(15) , 2644-57, (2007) In this second study on dual-chirality microemulsions containing a chiral surfactant and a chiral oil, a less hydrophobic and lower interfacial tension chiral oil, diethyl tartrate, is employed (Part 1, Foley, J. P. et al.., Electrophoresis, DOI: 10.1002/elps... |
|
|
Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.
Org. Lett. 13(4) , 616-9, (2011) A first total synthesis of broussonetine F from diethyl L-tartrate was achieved. The cornerstone of our synthesis was an orthoamide Overman rearrangement, which provided an allylic amino alcohol with complete diastereoselectivity. |
|
|
Influence of microemulsion chirality on chromatographic figures of merit in EKC: results with novel three-chiral-component microemulsions and comparison with one- and two-chiral-component microemulsions.
Electrophoresis 28(17) , 3024-40, (2007) Novel microemulsion formulations containing all chiral components are described for the enantioseparation of six pairs of pharmaceutical enantiomers (atenolol, ephedrine, metoprolol, N-methyl ephedrine, pseudoephedrine, and synephrine). The chiral surfactant ... |
|
|
Total syntheses of naturally occurring diacetylenic spiroacetal enol ethers.
J. Org. Chem. 70(15) , 6045-52, (2005) A highly stereoselective method for constructing a (2E)-methoxymethylidene-1,6-dioxaspiro[4.5]decane skeleton has been developed on the basis of the palladium(II)-catalyzed ring-closing reaction of the 3,4-dioxygenated-9-hydroxy-1-nonyn-5-one derivatives as a... |
|
|
Total synthesis of the light-harvesting carotenoid peridinin.
Angew. Chem. Int. Ed. Engl. 45(24) , 4023-7, (2006)
|
|
|
Pseudo-first-order alkaline hydrolysis of diethyl tartrate: a baseline study for a polymer matrix used in controlled-release delivery systems.
J. Pharm. Sci. 79(4) , 364-8, (1990) The hydrolysis kinetics of a bifunctional group compound, diethyl tartrate, was studied as a function of temperature and pH in the alkaline region. A pH-stat was used to maintain constant pH conditions in the alkaline region. This allowed the studies to be ca... |