| Structure | Name/CAS No. | Articles |
|---|---|---|
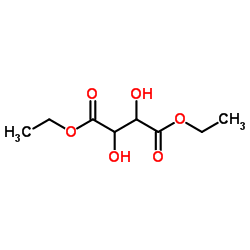 |
(-)-Diethyl D-tartrate
CAS:13811-71-7 |
|
 |
L(+)-Diethyl L-tartrate
CAS:87-91-2 |