n-trifluoroacetylimidazole
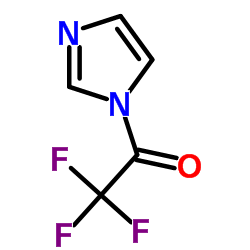
n-trifluoroacetylimidazole structure
|
Common Name | n-trifluoroacetylimidazole | ||
|---|---|---|---|---|
| CAS Number | 1546-79-8 | Molecular Weight | 164.085 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 151.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C5H3F3N2O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 45.0±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
|
Determination of mustard gas hydrolysis products thiodiglycol and thiodiglycol sulfoxide by gas chromatography-tandem mass spectrometry after trifluoroacetylation.
Anal. Chem. 86(12) , 5865-72, (2014) A method for detecting mustard gas degradation products thiodiglycol (TDG) and thiodiglycol sulfoxide (TDGO) in water and sediment samples using gas chromatography-tandem mass spectrometry (GC-MS/MS) after derivatization with 1-(trifluoroacetyl)imidazole (TFA... |
|
|
Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals.
J. Lipid Res. 48(4) , 816-25, (2007) Hydroxy-alkenals, such as 4-hydroxy-2(E)-nonenal (4-HNE; from n-6 fatty acids), are degradation products of fatty acid hydroperoxides, including those generated by free radical attack of membrane polyunsaturated fatty acyl moieties. The cytotoxic effects of h... |
|
|
The kynurenine metabolic pathway in the eye: studies on 3-hydroxykynurenine, a putative cataractogenic compound.
FEBS Lett. 453(1-2) , 197-200, (1999) The rabbit lens has an elevated content of 3-hydroxykynurenine (30HKYN) in spite of a very low activity of the enzymes leading to its synthesis. The iris/ciliary body, on the contrary, has very high activity of 30HKYN synthesizing enzymes but a content of 30H... |
|
|
J. Eagles and M.E. Knowles
Anal. Chem. 43 , 1697, (1971)
|
|
|
Direct microwave promoted trifluoroacetylation of aromatic amines with trifluoroacetic acid. Salazar J, et al.
J. Fluor. Chem. 124(1) , 111-13, (2003)
|
|
|
Trifluoroacetylation in Organic Synthesis: Reagents, Developments and Applications in the Construction of Trifluoromethylated Compounds. Lopez SE, et al.
Curr. Org. Synth. 7(5) , 414-32, (2010)
|