azetidinone
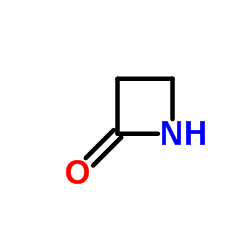
azetidinone structure
|
Common Name | azetidinone | ||
|---|---|---|---|---|
| CAS Number | 930-21-2 | Molecular Weight | 71.078 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 222.4±0.0 °C at 760 mmHg | |
| Molecular Formula | C3H5NO | Melting Point | 74-76 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 151.5±3.4 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
The influence of ezetimibe on classical and alternative activation pathways of monocytes/macrophages isolated from patients with hypercholesterolemia.
Naunyn Schmiedebergs Arch. Pharmacol. 387(8) , 733-42, (2014) Macrophages are crucial for the development of atherosclerotic plaques. Classically activated macrophages contribute to plaque growth and destabilization, while alternatively activated macrophages increase plaque stability. Here, we assessed the influence of ... |
|
|
Non-transpeptidase binding arylthioether β-lactams active against Mycobacterium tuberculosis and Moraxella catarrhalis.
Bioorg. Med. Chem. 23(3) , 632-47, (2015) The prevalence of drug resistance in both clinical and community settings as a consequence of alterations of biosynthetic pathways, enzymes or cell wall architecture is a persistent threat to human health. We have designed, synthesized, and tested a novel cla... |
|
|
Photoelectron spectra of some antibiotic building blocks: 2-azetidinone and thiazolidine-carboxylic acid.
J. Phys. Chem. A 116(33) , 8653-60, (2012) X-ray photoelectron spectra of the core and valence levels of the fundamental building blocks of β-lactam antibiotics have been investigated and compared with theoretical calculations. The spectra of the compounds 2-azetidinone and the 2- and 4-isomers of thi... |
|
|
Synthesis of optically pure highly functionalized gamma-lactams via 2-azetidinone-tethered iminophosphoranes.
J. Org. Chem. 69(3) , 993-6, (2004) A synthesis of optically pure densely functionalized gamma-lactams starting from 2-azetidinone-tethered iminophosphoranes has been developed. Full chirality transfer has been accomplished from the enantiomerically pure 2-azetidinones. The addition of lithium ... |
|
|
2-Azetidinone (ß-propiolactam) Holley RW and Holley AD.
J. Am. Chem. Soc. 71(6) , 2129-31, (1949)
|