fenazaquin
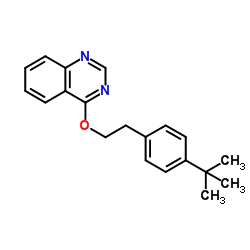
fenazaquin structure
|
Common Name | fenazaquin | ||
|---|---|---|---|---|
| CAS Number | 120928-09-8 | Molecular Weight | 306.401 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 461.0±33.0 °C at 760 mmHg | |
| Molecular Formula | C20H22N2O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 165.1±15.6 °C | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
|
Side-effects of fenazaquin on a cellular model of Paramecium.
Commun. Agric. Appl. Biol. Sci. 74(1) , 129-35, (2009) Our biodiversity has long been preserved, but the main constituents of our environment have been particularly affected by the addition of molecules resulting from agricultural and industrial activities. It is well accepted that these changes may stress some s... |
|
|
Investigation in tea on fate of fenazaquin residue and its transfer in brew.
Food Chem. Toxicol. 44(4) , 596-600, (2006) Fenazaquin is a non-systemic acaricide/insecticide used widely in controlling mites and other related pests in fruits, vegetables and tea. The objective of this research was to investigate the disappearance trend in tea of fenazaquin residue level and its tra... |
|
|
Incidence and inheritance of resistance to METI-acaricides in European strains of the two-spotted spider mite (Tetranychus urticae) (Acari: Tetranychidae).
Pest Manag. Sci. 57(5) , 443-8, (2001) A strain of Tetranychus urticae (Koch; Acari: Tetranychidae), collected from hops (Humulus humuli L; Cannabaceae) in England with a short history of tebufenpyrad use, exhibited resistance to four METI (mitochondrial electron transport inhibitor)-acaricides; t... |
|
|
NADH: ubiquinone oxidoreductase inhibitors block induction of ornithine decarboxylase activity in MCF-7 human breast cancer cells.
Pharmacol. Toxicol. 83(5) , 214-9, (1998) Rotenone is the classical inhibitor of NADH: ubiquinone oxidoreductase and its analogue deguelin is a potent inhibitor of 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced ornithine decarboxylase mRNA steady state level and enzyme activity in mouse 308 cells... |
|
|
Investigation in tea on fate of fenazaquin residue and its transfer in brew.
Food Chem. Toxicol. 42(3) , 423-8, (2004) Fenazaquin is a non-systemic acaricide/insecticide used widely in controlling mites and other related pests in fruits, vegetables and tea. The objective of this research was to investigate the disappearance trend in tea of fenazaquin residue level and its tra... |
|
|
Use of vancomycin hydrochloride for treatment of Clostridium difficile enteritis in Syrian hamsters.
Lab. Anim. Sci. 44(1) , 31-7, (1994) As part of an 18-month carcinogenicity study, 680 Syrian hamsters (Mesocricetus auratus) received daily gavage doses of fenazaquin, an experimental miticide. Mortality associated with severe enteritis was noticed beginning when the hamsters were 4 months old ... |
|
|
Photodecomposition of an acaricide, fenazaquin, in aqueous alcoholic solution.
J. Agric. Food Chem. 51(14) , 4013-6, (2003) Fenazaquin (I) is a new acaricide of the quinazoline class. The photodecomposition of I was studied in aqueous methanolic and 2-propanolic solution under UV light (30 h) and sunlight (70 h) separately. The photolytic half-lives in aqueous methanolic solution ... |
|
|
Synthesis and fungicidal activity of a series of novel aryloxylepidines.
Pest Manag. Sci. 57(9) , 844-51, (2001) A series of novel (hetero) aryloxylepidine derivatives was devised as hybrid structures of the phenoxyquinoline and phenethoxyquin(az)oline fungicides. Synthesis of these targets required the development of several new routes to derivatised 4-hydroxymethylqui... |
|
|
Regulatory implications of peroxisome proliferation: an industrial perspective.
Ann. N. Y. Acad. Sci. 804 , 641-8, (1996)
|
|
|
Effect of household processing on fenazaquin residues in okra fruits.
Bull. Environ. Contam. Toxicol. 84(2) , 217-20, (2010) Fenazaquin (4-[[4 (1,1-dimethylethyl) phenyl] ethoxy]quinazoline) is a new acaricide of the quinazoline class. Residue levels of fenazaquin were determined in unprocessed and processed okra fruits to evaluate the effect of different processes (washing, boilin... |