Beta-pinene
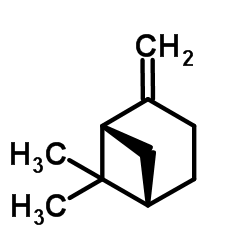
Beta-pinene structure
|
Common Name | Beta-pinene | ||
|---|---|---|---|---|
| CAS Number | 18172-67-3 | Molecular Weight | 136.234 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 166.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H16 | Melting Point | -61ºC | |
| MSDS | Chinese USA | Flash Point | 34.9±5.8 °C | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Danger | |
|
Antioxidant activity and cytotoxicity on tumour cells of the essential oil from Cedronella canariensis var. canariensis (L.) Webb & Berthel. (Lamiaceae).
Nat. Prod. Res. , 1-9, (2015) Cedronella canariensis is a lemon-scented species of the family Lamiaceae endemic to the Canary Islands where it is used in the traditional medicine to prepare infusions or inhalations for anti-catarrhal, tonic, diuretic, hypoglycaemiant, hypotensive, anti-in... |
|
|
Eucalyptol is an attractant of the Redbay ambrosia beetle, Xyleborus glabratus.
J. Chem. Ecol. 40(4) , 355-62, (2014) The redbay ambrosia beetle, Xyleborus glabratus, is an invasive wood-boring beetle that has become established in the southeastern United States. The beetle transmits the causal pathogen of lethal laurel wilt to susceptible host trees, which include redbay, a... |
|
|
Evidence for the existence of organosulfates from beta-pinene ozonolysis in ambient secondary organic aerosol.
Environ. Sci. Technol. 41(19) , 6678-83, (2007) The formation of organosulfates from the gas-phase ozonolysis of beta-pinene in the presence of neutral or acidic sulfate particles was investigated in a series of indoor aerosol chamber experiments. The organosulfates were analyzed using high-performance liq... |
|
|
Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils.
Chin. J. Nat. Med. 12(12) , 901-10, (2014) Essential oils of the resins of Pinus brutia and Pinus pinea were evaluated for their biological potential. Essential oils were characterized using GC-MS and GC/FID. in vitro antimicrobial, phytotoxic, antioxidant, and insecticidal activities were carried out... |
|
|
Aldrichimica Acta 13 , 13, (1980)
|
|
|
Tetrahedron Lett. 30 , 6653, (1989)
|
|
|
Modelling the formation and composition of secondary organic aerosol from α-and β-pinene ozonolysis using MCM v3. Jenkin M E.
Atmos. Chem. Phys. 4(7) , 1741-57, (2004)
|
|
|
Reversible in situ acid formation for ß-pinene hydrolysis using CO 2 expanded liquid and hot water. Chamblee TS, et al.
Green Chem. 6(8) , 382-6, (2004)
|
|
|
Hydroboration of Terpenes. II. The Hydroboration of α-and β-Pinene-The Absolute Configuration of the Dialkylborane from the Hydroboration of α-Pinene. Zweifel G and Brown HC.
J. Am. Chem. Soc. 86(3) , 393-397, (1964)
|
|
|
Reactions of ozone with α-pinene and β-pinene in air: Yields of gaseous and particulate products. Hatakeyama S, et al.
J. Geophys. Res. Atmos. 94(D10) , 13013-13024, (1989)
|