2-furaldehyde dimethylhydrazone
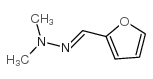
2-furaldehyde dimethylhydrazone structure
|
Common Name | 2-furaldehyde dimethylhydrazone | ||
|---|---|---|---|---|
| CAS Number | 14064-21-2 | Molecular Weight | 138.16700 | |
| Density | 1.042 g/mL at 25ºC(lit.) | Boiling Point | 98ºC 9.5 mm Hg(lit.) ,225ºC at 760 mmHg | |
| Molecular Formula | C7H10N2O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 90.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Further studies on the mechanism of phenol-sulfuric acid reaction with furaldehyde derivatives.
Anal. Biochem. 189(2) , 178-81, (1990) Even though the chromogens formed from mannose and galactose showed comparable absorbances at 480 nm in the conventional (developer present during heat of dilution) and modified (developer reacted at room temperature after cooling; epsilon mannose = 13,700, g... |
|
|
An in vitro comparative study with furyl-1,4-quinones endowed with anticancer activities.
Invest. New Drugs 29(5) , 760-7, (2011) We describe the biological activity of some furylbenzo- and naphthoquinones (furylquinones) on hepatocarcinoma cells and healthy rat liver slices. The effects of furylquinones on cancer cells (Transplantable Liver Tumor, TLT) were assessed by measuring cell d... |
|
|
Studies on quinones. Part 42: Synthesis of furylquinone and hydroquinones with antiproliferative activity against human tumor cell lines.
Bioorg. Med. Chem. 16(2) , 862-8, (2008) The preparation of furyl-1,4-quinone and hydroquinones by reaction of 2-furaldehyde N,N-dimethylhydrazone with benzo- and naphthoquinones is reported. Access to furylnaphthoquinones from unactivated quinones requires acid-induced conditions, however oxidative... |
|
|
Structure and UV-induced photochemistry of 2-furaldehyde dimethylhydrazone isolated in rare gas matrices.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 97 , 830-7, (2012) In this work, a combined matrix isolation FTIR and theoretical DFT(B3LYP)/6-311++G(d,p) study of 2-furaldehyde dimethylhydrazone (2FDH) was performed. According to calculations, two E and two Z conformers exist, the E forms having considerably lower energy th... |