Hexachlorophene
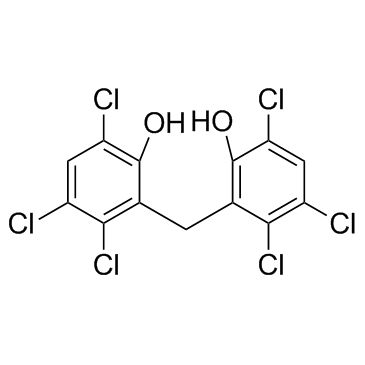
Hexachlorophene structure
|
Common Name | Hexachlorophene | ||
|---|---|---|---|---|
| CAS Number | 70-30-4 | Molecular Weight | 406.904 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 470.9±40.0 °C at 760 mmHg | |
| Molecular Formula | C13H6Cl6O2 | Melting Point | 163-165 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 238.6±27.3 °C | |
| Symbol |




GHS02, GHS06, GHS08, GHS09 |
Signal Word | Danger | |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Surfactants, aromatic and isoprenoid compounds, and fatty acid biosynthesis inhibitors suppress Staphylococcus aureus production of toxic shock syndrome toxin 1.
Antimicrob. Agents Chemother. 53 , 1898-906, (2009) Menstrual toxic shock syndrome is a rare but potentially life-threatening illness manifest through the actions of Staphylococcus aureus toxic shock syndrome toxin 1 (TSST-1). Previous studies have shown that tampon additives can influence staphylococcal TSST-... |
|
|
Identification and characterization of novel sirtuin inhibitor scaffolds.
Bioorg. Med. Chem. 17 , 7031-41, (2009) The sirtuin proteins are broadly conserved NAD(+)-dependent deacetylases that are implicated in diverse biological processes including DNA recombination and repair, transcriptional silencing, longevity, apoptosis, axonal protection, insulin signaling, and fat... |
|
|
Artemisinins, new miconazole potentiators resulting in increased activity against Candida albicans biofilms.
Antimicrob. Agents Chemother. 59(1) , 421-6, (2014) Mucosal biofilm-related fungal infections are very common, and the incidence of recurrent oral and vulvovaginal candidiasis is significant. As resistance to azoles (the preferred treatment) is occurring, we aimed at identifying compounds that increase the act... |
|
|
Biochemical and structural characterization of Plasmodium falciparum glutamate dehydrogenase 2.
Mol. Biochem. Parasitol. 183(1) , 52-62, (2012) Glutamate dehydrogenases (GDHs) play key roles in cellular redox, amino acid, and energy metabolism, thus representing potential targets for pharmacological interventions. Here we studied the functional network provided by the three known glutamate dehydrogen... |
|
|
Novel inhibitors complexed with glutamate dehydrogenase: allosteric regulation by control of protein dynamics.
J. Biol. Chem. 284(34) , 22988-3000, (2009) Mammalian glutamate dehydrogenase (GDH) is a homohexameric enzyme that catalyzes the reversible oxidative deamination of l-glutamate to 2-oxoglutarate using NAD(P)(+) as coenzyme. Unlike its counterparts from other animal kingdoms, mammalian GDH is regulated ... |
|
|
Hexachlorophene and cuprizone induce the spongy change of the developing rat brain by different mechanisms: the role of 2', 3'-cyclic nucleotide 3'-phosphodiesterase (CNPase).
J. Vet. Med. Sci. 74(7) , 837-43, (2012) The goal of this research was to identify mechanisms responsible for the spongy change induced in rats after repeated hexachlorophene (HCP) or cuprizone (CPZ) dosing. Rats were dosed with 35 mg/kg HCP for 5 days followed by drug withdrawal for 7 days suffered... |