oxamide
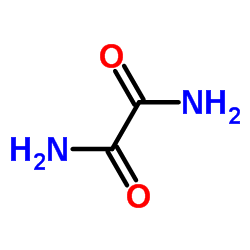
oxamide structure
|
Common Name | oxamide | ||
|---|---|---|---|---|
| CAS Number | 471-46-5 | Molecular Weight | 88.065 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 262.7±23.0 °C at 760 mmHg | |
| Molecular Formula | C2H4N2O2 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 112.7±22.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Uses of 1-(3-Cyano-4,5,6,7-tetrahydrobenzo[b]-thiophen-2-yl)-3-dodecanoylthiourea as a Building Block in the Synthesis of Fused Pyrimidine and Thiazine Systems.
Chem. Pharm. Bull. 63 , 450-6, (2015) The reaction of lauroyl isothiocyanate and 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile was used to synthesize the title compound 2. Compound 2 could serve as the main building block in the synthesis of many target heterocyclic systems. Various ... |
|
|
Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis.
Am. J. Pathol. 185(4) , 969-86, (2015) Myofibroblasts are crucial to the pathogenesis of tissue fibrosis. Their formation of stress fibers results in the release of myocardin-related transcription factor (MRTF), a transcriptional coactivator of serum response factor (SRF). MRTF-A (Mkl1)-deficient ... |
|
|
Antibacterial Schiff bases of oxalyl-hydrazine/diamide incorporating pyrrolyl and salicylyl moieties and of their zinc(II) complexes.
J. Enzyme Inhib. Med. Chem. 17(1) , 1-7, (2002) Schiff bases derived from oxaldiamide/oxalylhydrazine and pyrrol-2-carbaldehyde, or salicylaldehyde respectively, as well as their Zn(II) complexes have been prepared and tested as antibacterial agents. These Schiff bases function as tetradentate ligands, for... |
|
|
Discovery of indole tetrafluorophenoxymethylketone-based potent novel small molecule inhibitors of caspase-3.
Org. Med. Chem. Lett. 2 , 27, (2012) Caspase-3 inhibition has been demonstrated to be therapeutically effective in moderating excessive programmed cell death. Interest in caspase-3 as a therapeutic target has led many to pursue the development of inhibitors. To date, only a few series of non-pep... |
|
|
The determination of oxalic acid, oxamic acid, and oxamide in a drug substance by ion-exclusion chromatography.
J. Pharm. Biomed. Anal. 22(3) , 487-93, (2000) Oxalic acid, oxamic acid and oxamide are potential impurities in some active pharmaceutical ingredients (API). The retention and separation of oxalic and oxamic acids are particularly challenging using conventional reversed-phase HPLC due to their high polari... |
|
|
Synthesis of N-glucopyranosidic derivatives as potential inhibitors that bind at the catalytic site of glycogen phosphorylase.
Mini Rev. Med. Chem. 10(12) , 1127-38, (2010) Glycogen phosphorylase (GP) is a promising molecular target for the treatment of Type 2 diabetes. The design of potential inhibitors for the catalytic site of the enzyme is based on the high affinity for β-D-glucopyranose and the presence of a β-cavity that e... |
|
|
Crystal and molecular structures of related nickel(II) complexes of open-chain and macrocyclic oxamide-based ligands and the peculiarities of water aggregates in their crystal lattices.
Dalton Trans. (41) , 4708-14, (2007) A comparison of the molecular structure of related nickel(II) complexes of the open-chain and 13-membered macrocyclic oxamide-derived ligands NiL(1).4H2O and NiL(2).3H2O revealed that the formation of an additional 6-membered chelate ring in the complex resul... |
|
|
Physicobiological properties and biocompatibility of biodegradable poly(oxalate-co-oxamide).
J. Biomed. Mater. Res. A 98(4) , 517-26, (2011) The development of biodegradable and biocompatible materials is the basis for tissue engineering and drug delivery. The aims of this study are to develop the poly(oxalate-co-oxamide) (POXAM) and evaluate its physicochemical properties and biocompatibility as ... |
|
|
Selectively light scattering spectrometric detection of copper (II) based on a new synthesized oxamide ligand
Anal. Chim. Acta 624(1) , 128-32, (2008) Light scattering (LS) signals have been applied for analytical detections, but the selectivity is poor. In order to improve the selectivity, pre-separation or new machines are generally considered. Differing from these methods, we synthesized a highly selecti... |
|
|
Structural versatility of oxalamide-based compounds: a computational study on the isomerization of the oxalamide group and the structural preferences of the polyoxalamides.
J. Org. Chem. 66(24) , 8076-85, (2001) The conformational properties of the oxalamide group and crystal structure of several polyoxalamides have been investigated by computational methods. First, a detailed quantum mechanical study of the conformational preferences of N,N'-dimethyloxalamide is rep... |