p-Methoxybenzyl isocyanate
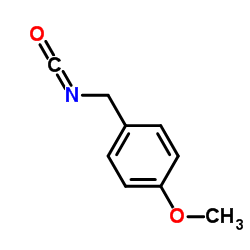
p-Methoxybenzyl isocyanate structure
|
Common Name | p-Methoxybenzyl isocyanate | ||
|---|---|---|---|---|
| CAS Number | 56651-60-6 | Molecular Weight | 163.173 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 249.9±23.0 °C at 760 mmHg | |
| Molecular Formula | C9H9NO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 112.6±17.1 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
|
Discovery of nitroaryl urea derivatives with antiproliferative properties.
J. Enzyme Inhib. Med. Chem. , 1-11, (2015) A series of urea derivatives bearing nitroaryl moiety has been synthesized and assayed for their potential antiproliferative activities. Some of the tested compounds displayed activity in RK33 laryngeal cancer cells and TE671 rhabdomyosarcoma cells while bein... |
|
|
The first synthesis of a novel 5:7:5-fused diimidazodiazepine ring system and some of its chemical properties.
Org. Lett. 10(20) , 4681-4, (2008) The first synthesis of a novel 5:7:5-fused heterocyclic ring system, a diimidazodiazepine, is reported. The propensity of the ring system to undergo facile, acid-catalyzed nucleophilic addition reactions by neutral carbon and nitrogen nucleophiles has been ex... |
|
|
Total synthesis of (+)-batzelladine A and (-)-batzelladine D via [4 + 2]-annulation of vinyl carbodiimides with N-alkyl imines.
J. Am. Chem. Soc. 128 , 13255, (2006) A diastereoselective [4 + 2]-annulation of vinyl carbodiimides with chiral N-alkyl imines has been developed to access the stereochemically rich polycyclic guanidine cores of the batzelladine alkaloids. Application of this strategy, together with additional k... |
|
|
Yaws CL.
Thermophysical Properties of Chemicals and Hydrocarbons , (2014), 567
|
|
|
Synthesis and herbicidal activity of opened hydantoin-ring derivatives of hydantocidin. Hiromi S, et al.
Biosci. Biotechnol. Biochem. 60(7) , 1198-1200, (1996)
|