Gabapentin
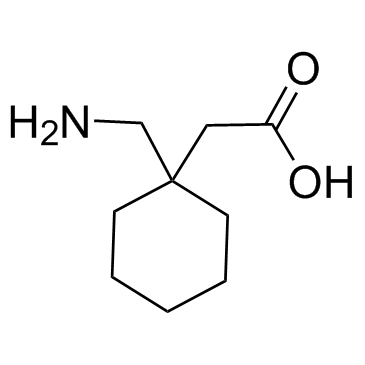
Gabapentin structure
|
Common Name | Gabapentin | ||
|---|---|---|---|---|
| CAS Number | 60142-96-3 | Molecular Weight | 171.237 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 314.4±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H17NO2 | Melting Point | 162°C | |
| MSDS | Chinese USA | Flash Point | 144.0±20.4 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Neural computational prediction of oral drug absorption based on CODES 2D descriptors.
Eur. J. Med. Chem. 45 , 930-40, (2010) A neural model based on a numerical molecular representation using CODES program to predict oral absorption of any structure is described. This model predicts both high and low-absorbed compounds with a global accuracy level of 74%. CODES/ANN methodology show... |
|
|
Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1.
J. Med. Chem. 51 , 5932-42, (2008) The liver-specific organic cation transport protein (OCT1; SLC22A1) transports several cationic drugs including the antidiabetic drug metformin and the anticancer agents oxaliplatin and imatinib. In this study, we explored the chemical space of registered ora... |
|
|
QSAR-based permeability model for drug-like compounds.
Bioorg. Med. Chem. 19 , 2615-24, (2011) A QSAR model was developed for predicting intestinal drug permeability, one of the most important parameters when evaluating compounds in drug discovery projects. First, a set of relevant properties for establishing a drug-like chemical space was applied to a... |
|
|
Hologram QSAR model for the prediction of human oral bioavailability.
Bioorg. Med. Chem. 15 , 7738-45, (2007) A drug intended for use in humans should have an ideal balance of pharmacokinetics and safety, as well as potency and selectivity. Unfavorable pharmacokinetics can negatively affect the clinical development of many otherwise promising drug candidates. A varie... |
|
|
Structural insights into thyroid hormone transport mechanisms of the L-type amino acid transporter 2.
Mol. Endocrinol. 29 , 933-42, (2015) Thyroid hormones (THs) are transported across cell membranes by different transmembrane transporter proteins. In previous studies, we showed marked 3,3'-diiodothyronine (3,3'-T2) but moderate T3 uptake by the L-type amino acid transporter 2 (Lat2). We have no... |
|
|
Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds.
Drug Metab. Dispos. 36 , 1385-405, (2008) We present herein a compilation and trend analysis of human i.v. pharmacokinetic data on 670 drugs representing, to our knowledge, the largest publicly available set of human clinical pharmacokinetic data. This data set provides the drug metabolism scientist ... |