Ceftiofur HCl
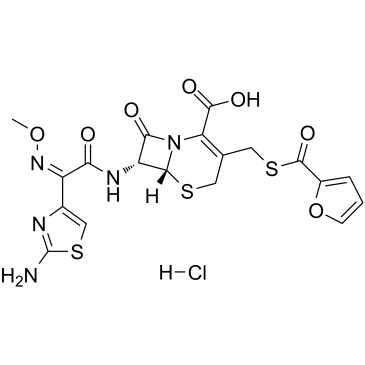
Ceftiofur HCl structure
|
Common Name | Ceftiofur HCl | ||
|---|---|---|---|---|
| CAS Number | 103980-44-5 | Molecular Weight | 560.023 | |
| Density | N/A | Boiling Point | 814.1ºC at 760 mmHg | |
| Molecular Formula | C19H18ClN5O7S3 | Melting Point | >190°C (dec.) | |
| MSDS | USA | Flash Point | 446.1ºC | |
|
blaCTX-M-32 on an IncN plasmid in Escherichia coli from beef cattle in the United States.
Antimicrob. Agents Chemother. 57(2) , 1096-7, (2013)
|
|
|
Genetic merit for fertility traits in Holstein cows: V. Factors affecting circulating progesterone concentrations.
J. Dairy Sci. 97(9) , 5543-57, (2014) This study investigated the factors affecting circulating progesterone (P4) concentrations in cows with similar genetic merit for milk production traits, but with extremes of good (Fert+) or poor (Fert-) genetic merit for fertility traits. Study 1: 28 cows we... |
|
|
Lipase is essential for the study of in vitro release kinetics from organogels.
Mol. Pharm. 9(6) , 1803-11, (2012) In vitro drug release studies remain indispensable in the development of drug delivery systems, even if correlations between in vitro and in vivo results are often imperfect. In this work, an improved in vitro analysis method for studying in situ-forming lipi... |
|
|
Validation study of the BetaStar plus lateral flow assay for detection of beta-lactam antibiotics in milk.
J. AOAC Int. 95(4) , 1211-21, (2012) A validation study designed to meet the requirements of the AOAC Research Institute and the U.S. Food and Drug Administration, Center for Veterinary Medicine (FDA/CVM) was conducted for a receptor and antibody-based, immunochromatographic method (BetaStar Plu... |
|
|
Comparison of enrofloxacin and ceftiofur sodium for the treatment of relapse of undifferentiated fever/bovine respiratory disease in feedlot cattle.
Can. Vet. J. 53(1) , 57-62, (2012) This commercial field trial compared the efficacy of enrofloxacin and ceftiofur sodium in beef cattle at high risk of developing undifferentiated fever (UF), also known as bovine respiratory disease (BRD) that received tilmicosin at feedlot arrival, were diag... |
|
|
Pharmacokinetics of a single intramuscular injection of ceftiofur crystalline-free acid in American black ducks (Anas rubripes).
Am. J. Vet. Res. 73(5) , 620-7, (2012) To determine the pharmacokinetic properties of 1 IM injection of ceftiofur crystalline-free acid (CCFA) in American black ducks (Anas rubripes).20 adult American black ducks (6 in a preliminary experiment and 14 in a primary experiment).Dose and route of admi... |
|
|
A determinative and confirmatory method for ceftiofur metabolite desfuroylceftiofur cysteine disulfide in bovine kidney by LC–MS/MS
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 898 , 62-8, (2012) Highlights ► We developed a new LC–MS/MS method for ceftiofur metabolite in bovine kidney. ► The new method is both determinative and confirmatory. ► The method utilizes a simple and effective extraction procedure. ► A deuterated internal standard was synthes... |
|
|
Disposition of desfuroylceftiofur acetamide in serum, placental tissue, fetal fluids, and fetal tissues after administration of ceftiofur crystalline free acid (CCFA) to pony mares with placentitis.
J. Vet. Pharmacol. Ther. 36(1) , 59-67, (2013) The objective of this study was to determine the pharmacokinetics of CCFA in mares with placentitis and evaluate the disposition of the drug in fetal fluids, fetal membranes, colostrum, and serum of foals. A secondary objective was to obtain pilot data regard... |
|
|
Pharmacokinetics of a long-acting ceftiofur crystalline-free acid formulation in Asian elephants (Elephas maximus).
Am. J. Vet. Res. 73(10) , 1512-8, (2012) To determine the pharmacokinetics of a long-acting formulation of ceftiofur, ceftiofur crystalline-free acid (CCFA), following SC injection to Asian elephants (Elephas maximus).11 adult Asian elephants.Each elephant received CCFA (6.6 mg/kg, SC) in the area c... |
|
|
Urine from treated cattle drives selection for cephalosporin resistant Escherichia coli in soil.
PLoS ONE 7(11) , e48919, (2012) The U.S. Food and Drug Administration recently issued new rules for using ceftiofur in food animals in part because of an increasing prevalence of enteric bacteria that are resistant to 3(rd)-generation cephalosporins. Parenteral ceftiofur treatment, however,... |