Clofarabine
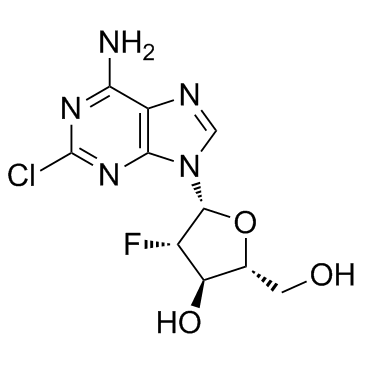
Clofarabine structure
|
Common Name | Clofarabine | ||
|---|---|---|---|---|
| CAS Number | 123318-82-1 | Molecular Weight | 303.677 | |
| Density | 2.1±0.1 g/cm3 | Boiling Point | 599.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C10H11ClFN5O3 | Melting Point | 228-231 °C | |
| MSDS | Chinese USA | Flash Point | 316.4±32.9 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
The potential of clofarabine in MLL-rearranged infant acute lymphoblastic leukaemia.
Eur. J. Cancer 51 , 2008-21, (2015) MLL-rearranged acute lymphoblastic leukaemia (ALL) in infants is the most difficult-to-treat type of childhood ALL, displaying a chemotherapy-resistant phenotype, and unique histone modifications, gene expression signatures and DNA methylation patterns. MLL-r... |
|
|
Preclinical examination of clofarabine in pediatric ependymoma: intratumoral concentrations insufficient to warrant further study.
Cancer Chemother. Pharmacol. 75 , 897-906, (2015) Clofarabine, a deoxyadenosine analog, was an active anticancer drug in our in vitro high-throughput screening against mouse ependymoma neurospheres. To characterize the clofarabine disposition in mice for further preclinical efficacy studies, we evaluated the... |
|
|
Knockdown of Bcl-xL enhances growth-inhibiting and apoptosis-inducing effects of resveratrol and clofarabine in malignant mesothelioma H-2452 cells.
J. Korean Med. Sci. 29(11) , 1464-72, (2014) Mcl-1 and Bcl-xL, key anti-apoptotic proteins of the Bcl-2 family, have attracted attention as important molecules in the cell survival and drug resistance. In this study, we investigated whether inhibition of Bcl-xL influences cell growth and apoptosis again... |
|
|
Clofarabine in combination with a standard remission induction regimen (cytosine arabinoside and idarubicin) in patients with previously untreated intermediate and bad-risk acute myelogenous leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS): phase I results of an ongoing phase I/II study of the leukemia groups of EORTC and GIMEMA (EORTC GIMEMA 06061/AML-14A trial).
Ann. Hematol. 93(6) , 965-75, (2014) This study aims to determine the maximum tolerated dose (MTD) of clofarabine combined with the EORTC-GIMEMA 3 + 10 induction regimen (idarubicin + cytosine arabinoside) in adults with untreated acute myelogenous leukemia or high-risk myelodysplastic syndrome.... |
|
|
Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients ≤60 years with newly diagnosed acute myeloid leukemia.
Am. J. Hematol. 88(11) , 961-6, (2013) Clofarabine is a second generation nucleoside analogue with activity in adults with acute myeloid leukemia (AML). A phase I trial of clofarabine, idarubicin, and cytarabine (CIA) in relapsed and refractory AML had shown an overall response rate (ORR) of 48%. ... |
|
|
Comparison of the cytotoxicity of cladribine and clofarabine when combined with fludarabine and busulfan in AML cells: Enhancement of cytotoxicity with epigenetic modulators.
Exp. Hematol. 43 , 448-61.e2, (2015) Clofarabine (Clo), fludarabine (Flu), and busulfan (Bu) combinations are efficacious in hematopoietic stem cell transplantation for myeloid leukemia. We sought to determine whether the more affordable drug cladribine (Clad) can provide a viable alternative to... |
|
|
Melatonin overcomes resistance to clofarabine in two leukemic cell lines by increased expression of deoxycytidine kinase.
Exp. Hematol. 43(3) , 207-14, (2015) Drug resistance remains a serious problem in leukemia therapy. Among newly developed nucleoside antimetabolites, clofarabine has broad cytotoxic activity showing therapeutic promise and is currently approved for relapsed acute lymphoblastic leukemia. To inves... |
|
|
Clofarabine in combination with pegylated asparaginase in the frontline treatment of childhood acute lymphoblastic leukaemia: a feasibility report from the CoALL 08-09 trial.
Br. J. Haematol. 163(2) , 240-7, (2013) Clofarabine was the latest new drug to be approved, in 2004, for relapsed or refractory acute lymphoblastic leukaemia (ALL). To investigate its value in the frontline treatment of ALL we applied clofarabine 5 × 40 mg/m(2) in combination with pegylated asparag... |
|
|
A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy.
Mol. Ther. 23 , 1507-18, (2015) The adoptive transfer of chimeric antigen receptor (CAR) T cell represents a highly promising strategy to fight against multiple cancers. The clinical outcome of such therapies is intimately linked to the ability of effector cells to engraft, proliferate, and... |
|
|
Phase II trial of clofarabine and daunorubicin as induction therapy for acute myeloid leukemia patients greater than or equal to 60 years of age.
Leuk. Res. 37(11) , 1468-71, (2013) We designed a phase II study evaluating the upfront combination of clofarabine and daunorubicin in acute myeloid leukemia (AML) patients60 years old. The median age of the 21 patients was 69 (range 60-85) years. Fourteen patients (67%) had unfavorable risk fe... |