4-Pentenoic anhydride
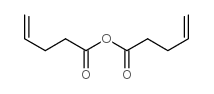
4-Pentenoic anhydride structure
|
Common Name | 4-Pentenoic anhydride | ||
|---|---|---|---|---|
| CAS Number | 63521-92-6 | Molecular Weight | 182.21600 | |
| Density | 0.997 g/mL at 25ºC(lit.) | Boiling Point | 78-81ºC0.4 mm Hg(lit.) | |
| Molecular Formula | C10H14O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 230 °F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Surface Eroding, Semicrystalline Polyanhydrides via Thiol-Ene "Click" Photopolymerization.
Biomacromolecules 16 , 1650-9, (2015) Surface eroding and semicrystalline polyanhydrides, with tunable erosion times and drug delivery pharmacokinetics largely dictated by erosion, are produced easily with thiol-ene "click" polymerization. This strategy yields both linear and cross-linked network... |
|
|
Photopolymerized cross-linked thiol-ene polyanhydrides: erosion, release, and toxicity studies.
Biomacromolecules 15(7) , 2573-82, (2014) Several critical aspects of cross-linked polyanhydrides made using thiol-ene polymerization are reported, in particular the erosion, release, and solution properties, along with their cytotoxicity toward fibroblast cells. The monomers used to synthesize these... |
|
|
Thiol-yne and thiol-ene "click" chemistry as a tool for a variety of platinum drug delivery carriers, from statistical copolymers to crosslinked micelles.
Biomacromolecules 12(5) , 1738-51, (2011) Statistical and block copolymers based on poly(2-hydroxyethyl methacrylate) (PHEMA) and poly[oligo(ethylene glycol) methylether methacrylate] (POEGMEMA) were modified with 4-pentenoic anhydride or 4-oxo-4-(prop-2-ynyloxy)butanoic anhydride to generate polymer... |
|
|
Synthesis of thiol-linked neoglycopolymers and thermo-responsive glycomicelles as potential drug carrier.
Chem. Commun. (Camb.) (10) , 1198-200, (2009) Homopolymer and block copolymer bearing carbohydrate side chain functionality were obtained by grafting glucothiose onto alkene functional scaffolds via a thiol-ene click reaction and the resulting copolymer was used to form thermo-responsive micelles as a po... |
|
|
Studies of the mechanism of the hypoglycemic action of 4-pentenoic acid. Corredor C, et al.
Proc. Natl. Acad. Sci. U. S. A. 58(6) , 2299, (1967)
|
|
|
2-(Trimethylsilyl) ethyl Glycosides. Transformation into the Corresponding 1-O-Acyl Sugars. Ellervik U and Magnusson G.
Acta Chem. Scand. 47 , 826, (1993)
|