2-Methylanisole
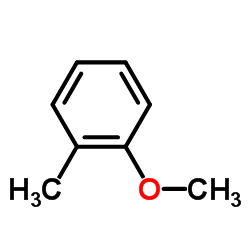
2-Methylanisole structure
|
Common Name | 2-Methylanisole | ||
|---|---|---|---|---|
| CAS Number | 578-58-5 | Molecular Weight | 122.164 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 171.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H10O | Melting Point | 170-172 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 51.7±0.0 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
|
Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry.
Phytochemistry 109 , 66-75, (2014) Frankincense has been known, traded and used throughout the ages for its exceptional aroma properties, and is still commonly used in both secular and religious settings to convey a pleasant odor. Surprisingly, the odoriferous principle(s) underlying its uniqu... |
|
|
An assessment of the reaction energetics for cytochrome P450-mediated reactions.
Arch. Biochem. Biophys. 385(1) , 220-30, (2001) Regioselectivity is used to determine the absolute energetic differences for four different reactions catalyzed by P450. Abstraction of a hydrogen from a benzylic carbon containing a chlorine has a 1.0 kcal/mol lower barrier than abstraction from a simple ben... |
|
|
(+)- and (-)-mutisianthol: first total synthesis, absolute configuration, and antitumor activity.
J. Org. Chem. 74(6) , 2561-6, (2009) The first synthesis of the natural product (+)-mutisianthol was accomplished in 11 steps and in 21% overall yield from 2-methylanisole. The synthesis of its enantiomer was also performed in a similar overall yield. The absolute configuration of the sesquiterp... |
|
|
Chiral ammonium-capped rhodium(0) nanocatalysts: synthesis, characterization, and advances in asymmetric hydrogenation in neat water.
ChemSusChem 5(1) , 91-101, (2012) Optically active amphiphilic compounds derived from N-methylephedrine, N-methylprolinol, or cinchona derivatives possessing bromide or chiral lactate counterions were efficiently used as protective agents for rhodium(0) nanoparticles. The full characterizatio... |