7,8-Benzoflavone
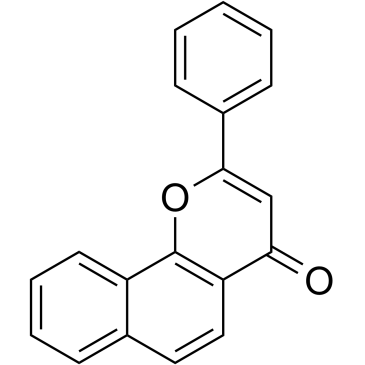
7,8-Benzoflavone structure
|
Common Name | 7,8-Benzoflavone | ||
|---|---|---|---|---|
| CAS Number | 604-59-1 | Molecular Weight | 272.297 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 460.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H12O2 | Melting Point | 153-157 °C(lit.) | |
| MSDS | USA | Flash Point | 215.8±22.3 °C | |
|
Development and validation of a liquid-chromatography high-resolution tandem mass spectrometry approach for quantification of nine cytochrome P450 (CYP) model substrate metabolites in an in vitro CYP inhibition cocktail.
Anal. Bioanal. Chem 406(18) , 4453-64, (2014) Knowledge about the cytochrome P450 (CYP) inhibition potential of new drug candidates is important for drug development because of its risk of interactions. For novel psychoactive substances (NPS), corresponding data are not available. For developing a genera... |
|
|
In vitro cytochrome P450 inhibition potential of methylenedioxy-derived designer drugs studied with a two-cocktail approach.
Arch. Toxicol. 90 , 305-18, (2016) In vitro cytochrome P450 (CYP) inhibition assays are common approaches for testing the inhibition potential of drugs for predicting potential interactions. In contrast to marketed medicaments, drugs of abuse, particularly the so-called novel psychoactive subs... |
|
|
Cytochrome P450 inhibition potential of new psychoactive substances of the tryptamine class.
Toxicol. Lett. 241 , 82-94, (2015) New psychoactive substances (NPS) are not tested for their cytochrome P450 (CYP) inhibition potential before consumption. Therefore, this potential was explored for tryptamine-derived NPS (TDNPS) including alpha-methyl tryptamines (AMTs), dimethyl tryptamines... |
|
|
Uremic toxin indoxyl 3-sulfate regulates the differentiation of Th2 but not of Th1 cells to lessen allergic asthma.
Toxicol. Lett. 225(1) , 130-8, (2014) Immune system dysfunctions including the increased Th1/Th2 ratio are common in chronic kidney disease (CKD) patients, and a wide variety of skin diseases including Th1-mediated uremic pruritis are associated with CKD. Although there are more than 90 uremic to... |
|
|
Effects of cytochrome P450 inhibitors on peroxidase activity.
Neuro Endocrinol. Lett. 33 Suppl 3 , 33-40, (2012) Of several enzymes metabolizing xenobiotics, cytochrome P450 (CYP) and peroxidase enzymes seem to be most important. One of the major challenges in studies investigating metabolism of xenobiotics is to resolve which of these two groups of enzymes is predomina... |
|
|
Cytochrome p450 enzymes mechanism based inhibitors: common sub-structures and reactivity.
Curr. Drug Metab. 6 , 413-54, (2005) The inhibition of human cytochrome P450s (CYPs) is one of the most common mechanisms which can lead to drug-drug interactions. The inhibition of CYPs can be reversible (competitive or non-competitive) or irreversible. Irreversible inhibition usually derives f... |
|
|
OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice.
PLoS Med. 3 , e466, (2006) Tuberculosis (TB) is still a leading cause of death worldwide. Almost a third of the world's population is infected with TB bacilli, and each year approximately 8 million people develop active TB and 2 million die as a result. Today's TB treatment, which date... |
|
|
Pharmacophore modeling strategies for the development of novel nonsteroidal inhibitors of human aromatase (CYP19)
Bioorg. Med. Chem. Lett. 20 , 3050-64, (2010) The present study utilizes for the first time the structural information of aromatase, an important pharmacological target in anti-breast cancer therapy, to extract the pharmacophoric features important for interactions between the enzyme and its substrate, a... |
|
|
Hepatic cytochrome P450s attenuate the cytotoxicity induced by leflunomide and its active metabolite A77 1726 in primary cultured rat hepatocytes.
Toxicol. Sci. 122(2) , 579-86, (2011) The Black Box Warning section of the U.S. drug label for leflunomide was recently updated to include stronger warnings about potential hepatotoxicity from this novel anti-arthritis drug. Because metabolic activation is a key mechanism for drug-induced hepatot... |
|
|
Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development.
Bioorg. Med. Chem. 15 , 5047-60, (2007) Cytochrome P450s (CYPs) represent a large class of heme-containing enzymes that catalyze the metabolism of multitudes of substrates both endogenous and exogenous. Until recently, however, CYPs have been largely overlooked in cancer drug development, acknowled... |