Sclareol
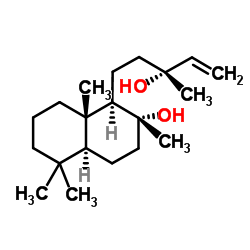
Sclareol structure
|
Common Name | Sclareol | ||
|---|---|---|---|---|
| CAS Number | 515-03-7 | Molecular Weight | 308.499 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 398.3±15.0 °C at 760 mmHg | |
| Molecular Formula | C20H36O2 | Melting Point | 95-100 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 169.1±15.0 °C | |
|
Parallel dual secondary column-dual detection: a further way of enhancing the informative potential of two-dimensional comprehensive gas chromatography.
J. Chromatogr. A. 1360 , 264-74, (2014) Comprehensive two-dimensional gas chromatography (GC×GC) coupled with Mass Spectrometry (MS) is one of today's most powerful analytical platforms for detailed analysis of medium-to-high complexity samples. The column set usually consists of a long, convention... |
|
|
Labdane diterpenoids from Salvia reuterana.
Phytochemistry 108 , 264-9, (2014) Three labdane diterpenoids, 14α-hydroxy-15-chlorosclareol (1), 14α-hydroxy-15-acetoxysclareol (2), and 6β-hydroxy-14α-epoxysclareol (3), together with the known diterpenoids sclareol (4), 6β-hydroxysclareol (5), and 14α-epoxysclareol (6), as well as other com... |
|
|
Isolation, chemical, and biotransformation routes of labdane-type diterpenes.
Chem. Rev. 111(8) , 4418-52, (2011)
|
|
|
Bioactive constituents of Salvia chrysophylla Stapf.
Nat. Prod. Res. 27(4-5) , 438-47, (2013) The dichloromethane extract of the aerial parts of Salvia chrysophylla Stapf (Lamiaceae), which is an endemic species to south-western Anatolia, was studied for non-volatile secondary metabolites for the first time in this study. Structures of the isolated co... |
|
|
Hemisynthesis of new labdane derivatives from (-)-sclareol.
Nat. Prod. Res. 20(10) , 887-95, (2006) A straightforward and very simple access to different analogues of (+)-subersic acid 7, the unnatural enantiomer (compounds 12) from naturally occurring (-)-sclareol 1 is described. We also report new conditions for the preparation of keto-intermediate 8 usin... |
|
|
The labdane diterpene sclareol (labd-14-ene-8, 13-diol) induces apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53-independent mechanism of action.
Eur. J. Pharmacol. 666(1-3) , 173-82, (2011) The labdane diterpene sclareol has demonstrated significant cytotoxicity against human tumor cell lines and human colon cancer xenografts. Therefore, there is need to elucidate the mode of action of this compound as very little information is known for the an... |
|
|
Synthesis of the scalarane sesterterpenoid 16-deacetoxy-12-epi-scalarafuranacetate.
J. Org. Chem. 76(17) , 7216-21, (2011) The marine natural product 16-deacetoxy-12-epi-scalarafuranacetate, isolated from Spongua officinalis , was synthesized in 18 linear steps, starting from (-)-sclareol, with high stereoselectivity and an overall yield of 6.1%. The intermediate 16-deacetoxy-12-... |
|
|
Extracellular localization of the diterpene sclareol in clary sage (Salvia sclarea L., Lamiaceae).
PLoS ONE 7(10) , e48253, (2012) Sclareol is a high-value natural product obtained by solid/liquid extraction of clary sage (Salvia sclarea L.) inflorescences. Because processes of excretion and accumulation of this labdane diterpene are unknown, the aim of this work was to gain knowledge on... |
|
|
Fragrance material review on sclareol.
Food Chem. Toxicol. 46 Suppl 11 , S270-4, (2008) A toxicologic and dermatologic review of sclareol when used as a fragrance ingredient is presented. |
|
|
Structure elucidation and antibacterial activity of new fungal metabolites of sclareol.
Chem. Biodivers. 3(1) , 54-61, (2006) The transformation of the antibacterial diterpene sclareol (1) by two different fungal strains was investigated (Scheme). In the presence of Rhizopus stolonifer, (3beta)-3-hydroxysclareol (2), 18-hydroxysclareol (3), (6alpha)-6,18-dihydroxysclareol (4), and (... |