Journal of Organic Chemistry
2011-09-02
Synthesis of the scalarane sesterterpenoid 16-deacetoxy-12-epi-scalarafuranacetate.
Xi-Bo Chen, Qian-Jia Yuan, Jing Wang, Si-Kai Hua, Jiangmeng Ren, Bu-Bing Zeng
Index: J. Org. Chem. 76(17) , 7216-21, (2011)
Full Text: HTML
Abstract
The marine natural product 16-deacetoxy-12-epi-scalarafuranacetate, isolated from Spongua officinalis , was synthesized in 18 linear steps, starting from (-)-sclareol, with high stereoselectivity and an overall yield of 6.1%. The intermediate 16-deacetoxy-12-epi-scalarafuran could be easily transformed into a series of natural scalarane sesterterpenoids in a few steps.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
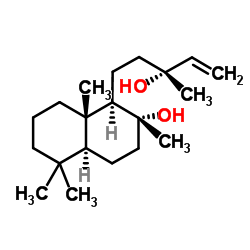 |
Sclareol
CAS:515-03-7 |
C20H36O2 |
Related Articles:
More...
|
Parallel dual secondary column-dual detection: a further way...
2014-09-19 [J. Chromatogr. A. 1360 , 264-74, (2014)] |
|
Labdane diterpenoids from Salvia reuterana.
2014-12-01 [Phytochemistry 108 , 264-9, (2014)] |
|
Isolation, chemical, and biotransformation routes of labdane...
2011-08-10 [Chem. Rev. 111(8) , 4418-52, (2011)] |
|
Bioactive constituents of Salvia chrysophylla Stapf.
2013-03-01 [Nat. Prod. Res. 27(4-5) , 438-47, (2013)] |
|
Hemisynthesis of new labdane derivatives from (-)-sclareol.
2006-08-01 [Nat. Prod. Res. 20(10) , 887-95, (2006)] |