8-Hydroxy-5-quinolinesulfonic acid
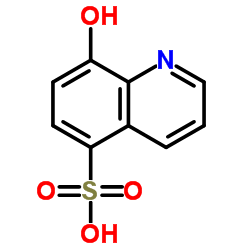
8-Hydroxy-5-quinolinesulfonic acid structure
|
Common Name | 8-Hydroxy-5-quinolinesulfonic acid | ||
|---|---|---|---|---|
| CAS Number | 84-88-8 | Molecular Weight | 225.221 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 432ºC | |
| Molecular Formula | C9H7NO4S | Melting Point | 311-313°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Potential application of N-carbamoyl-beta-alanine amidohydrolase from Agrobacterium tumefaciens C58 for beta-amino acid production.
Appl. Environ. Microbiol. 75(2) , 514-20, (2009) An N-carbamoyl-beta-alanine amidohydrolase of industrial interest from Agrobacterium tumefaciens C58 (beta car(At)) has been characterized. Beta car(At) is most active at 30 degrees C and pH 8.0 with N-carbamoyl-beta-alanine as a substrate. The purified enzym... |
|
|
NMR, DFT and luminescence studies of the complexation of Al(III) with 8-hydroxyquinoline-5-sulfonate.
Dalton Trans. 41(40) , 12478-89, (2012) Multinuclear ((1)H, (13)C and (27)Al) magnetic resonance spectroscopy (1D and 2D), DFT calculations and fluorescence have been used to study the complexation of 8-hydroxyquinoline-5-sulfonate (8-HQS) with Al(III). The study combines the high sensitivity of lu... |
|
|
Structural and photophysical studies on gallium(III) 8-hydroxyquinoline-5-sulfonates. Does excited state decay involve ligand photolabilisation?
Dalton Trans. 42(10) , 3682-94, (2013) Multinuclear ((1)H, (13)C and (71)Ga) magnetic resonance spectroscopy (1D and 2D), DFT calculations and luminescence techniques have been used to study 8-hydroxyquinoline-5-sulfonate (8-HQS) and its complexes with Ga(III) in aqueous solutions. The study combi... |
|
|
Self-assembled polymeric chelate nanoparticles as potential theranostic agents.
ChemPhysChem 13(18) , 4244-50, (2012) Improvements in cancer diagnostics and therapy have recently attracted the interest of many different branches of science. This study presents one of the new possible approaches in the diagnostics and therapy of cancer by using polymeric chelates as carriers.... |
|
|
Comparative solution equilibrium studies of anticancer gallium(III) complexes of 8-hydroxyquinoline and hydroxy(thio)pyrone ligands.
J. Inorg. Biochem. 117 , 189-97, (2012) The stoichiometry and stability constants of the Ga(III) complexes of 8-hydroxyquinoline (HQ), 8-hydroxyquinoline-5-sulfonate (HQS), maltol, thiomaltol, allomaltol and thioallomaltol were determined by means of pH-potentiometry, UV-vis spectrophotometry, spec... |
|
|
Absorption and excretion of organic compounds of copper by sheep.
J. Comp. Pathol. 93(4) , 551-8, (1983) When sheep are injected subcutaneously with copper calcium edetate or copper oxyquinoline sulphonate there is a rapid increase in the concentration of copper in whole blood, serum and urine within the first 24 h. When sheep are injected with copper methionate... |
|
|
Functional constituents of the active site of human neutrophil collagenase.
Arch. Biochem. Biophys. 246(2) , 645-9, (1986) A series of chemical modification reactions has been carried out to identify functional constituents of the active site of human neutrophil collagenase. The enzyme is reversibly inhibited by the transition metal chelating agent 1,10-phenanthroline, and inhibi... |
|
|
Bovine liver dihydropyrimidine amidohydrolase: pH dependencies of inactivation by chelators and steady-state kinetic properties.
Arch. Biochem. Biophys. 248(1) , 368-78, (1986) Dihydropyrimidine amidohydrolase (EC 3.5.2.2) catalyzes the reversible hydrolysis of 5,6-dihydropyrimidines to the corresponding beta-ureido acids. Previous work has shown that incubation of this Zn2+ metalloenzyme with 2,6-dipicolinic acid, 8-hydroxyquinolin... |
|
|
Synthesis and biological activity of some 5-substituted aminomethyl-8-hydroxyquinoline-7-sulphonic acids.
J. Chem. Technol. Biotechnol. 49(3) , 243-7, (1990) 5-Aryl (or alkyl)-8-hydroxyquinoline-7-sulphonic acids have been prepared by the Mannich reaction of 8-hydroxyquinoline-7-sulphonic acid with primary and secondary amines. Their bactericidal activities have been determined. |
|
|
Electrochemical spectroscopic investigations on the interaction of an ytterbium complex with DNA and their analytical applications such as biosensor.
Int. J. Biol. Macromol. 49(5) , 1117-23, (2011) Metal ion-DNA interactions are important in nature, often changing the genetic material's structure and function. A new Yb complex of YbCl(3) (tris(8-hydroxyquinoline-5-sulfonic acid) ytterbium) was synthesized and utilized as an electrochemical indicator for... |