RifamycinS
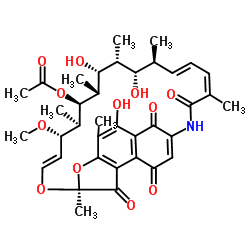
RifamycinS structure
|
Common Name | RifamycinS | ||
|---|---|---|---|---|
| CAS Number | 13553-79-2 | Molecular Weight | 695.753 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 917.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C37H45NO12 | Melting Point | 179-181ºC (dec.) | |
| MSDS | N/A | Flash Point | 508.6±34.3 °C | |
|
Polyketide construction via hydrohydroxyalkylation and related alcohol C-H functionalizations: reinventing the chemistry of carbonyl addition.
Nat. Prod. Rep. 31(4) , 504-13, (2014) Despite the longstanding importance of polyketide natural products in human medicine, nearly all commercial polyketide-based drugs are prepared through fermentation or semi-synthesis. The paucity of manufacturing routes involving de novo chemical synthesis re... |
|
|
Synthesis and structure-activity relationships of novel substituted 8-amino, 8-thio, and 1,8-pyrazole congeners of antitubercular rifamycin S and rifampin.
Bioorg. Med. Chem. Lett. 21 , 6094-9, (2011) A series of rifamycin S and rifampin analogues incorporating substituted 8-amino, 8-thio, and 1,8-pyrazole substituents has been synthesized. The compounds were made by activation of the C-8 phenol as a sulfonate ester, followed by displacement with selected ... |
|
|
Direct generation of acyclic polypropionate stereopolyads via double diastereo- and enantioselective iridium-catalyzed crotylation of 1,3-diols: beyond stepwise carbonyl addition in polyketide construction.
J. Am. Chem. Soc. 133(32) , 12795-800, (2011) Under the conditions of transfer hydrogenation employing the cyclometalated iridium catalyst (R)-I derived from [Ir(cod)Cl](2), allyl acetate, 4-cyano-3-nitrobenzoic acid, and the chiral phosphine ligand (R)-SEGPHOS, α-methylallyl acetate engages 1,3-propaned... |
|
|
Detoxification of toxins by bacillithiol in Staphylococcus aureus.
Microbiology 158(Pt 4) , 1117-26, (2012) Bacillithiol (BSH), an α-anomeric glycoside of l-cysteinyl-d-glucosaminyl-l-malate, is a major low-molecular-mass thiol found in bacteria such as Bacillus sp., Staphylococcus aureus and Deinococcus radiodurans. Like other low-molecular-mass thiols such as glu... |
|
|
Solid state cultivation of Curvularia lunata for transformation of rifamycin B to S.
Indian J. Exp. Biol. 40(8) , 930-3, (2002) Biotransformation of rifamycin B to rifamycin S using two strains of C. lunata namely NCIM 716 and NMU grown on various solid substrates viz., grass, paper, jowar/wheat straw, bran and bagasse was studied. Almost complete biotransformation efficiency of rifam... |
|
|
Studies on rifamycin oxidase immobilized on kappa-carrageenan gel.
Biomater. Artif. Cells Immobilization Biotechnol. 21(5) , 665-74, (1993) Rifamycin oxidase from Curvularia lunata var. aeria was immobilized on kappa-carrageenan gel where the enzyme showed excellent catalyzing activity and operational stability. Factors affecting the activity of immobilized enzyme preparation such as pH and tempe... |
|
|
Transformation of rifamycin S into rifamycins B and L. A revision of the current biosynthetic hypothesis.
J. Antibiot. 35(1) , 74-80, (1982) The transformation of rifamycin S into rifamycins B and L was reinvestigated in order to establish more detailed pathways. Our results exclude rifamycin O as a common progenitor in the biosyntheses of rifamycins B and L. Rifamycins B and L are formed from rif... |
|
|
Oxygen Enhancement of bactericidal activity of rifamycin SV on Escherichia coli and aerobic oxidation of rifamycin SV to rifamycin S catalyzed by manganous ions: the role of superoxide.
J. Biochem. 91(1) , 381-95, (1982) Oxygen enhanced the bactericidal activity of rifamycin SV to Escherichia coli K12. Anaerobically grown cells, which had a low level of superoxide dismutase, were more susceptible to the bactericidal activity than aerobically grown cells, which contained a hig... |
|
|
Molecular structure and conformation of rifamycin S, a potent inhibitor of DNA-dependent RNA polymerase.
J. Antibiot. 45(3) , 428-31, (1992)
|
|
|
NMR spectroscopic analysis of new spiro-piperidylrifamycins.
Magn. Reson. Chem. 43(4) , 269-82, (2005) New spiro-piperidylrifamycin derivatives are presented. These compounds were synthesized from the reaction of 3-amino-4-iminorifamycin S and enantiomerically pure 4-piperidones, which generate diasteroisomeric rifabutin analogues with a new stereocentre at th... |