| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Erythromycin
CAS:114-07-8 |
|
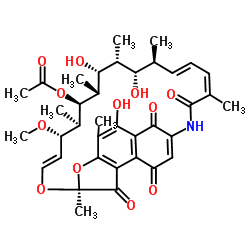 |
RifamycinS
CAS:13553-79-2 |
|
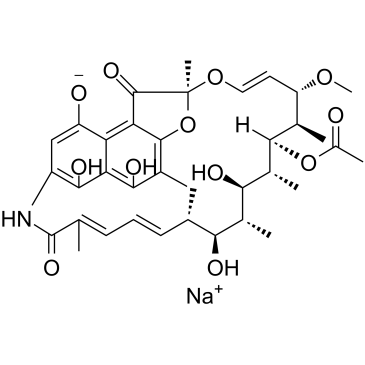 |
Rifamycin sodium
CAS:14897-39-3 |