715567
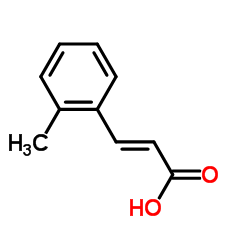
715567 structure
|
Common Name | 715567 | ||
|---|---|---|---|---|
| CAS Number | 2373-76-4 | Molecular Weight | 162.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 293.5±9.0 °C at 760 mmHg | |
| Molecular Formula | C10H10O2 | Melting Point | 174-176 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 199.4±9.6 °C | |
|
Walphos versus Biferrocene-Based Walphos Analogues in the Asymmetric Hydrogenation of Alkenes and Ketones.
Organometallics 33(8) , 1945-1952, (2014) Two representative Walphos analogues with an achiral 2,2_-biferrocenediyl backbone were synthesized. These diphosphine ligands were tested in the rhodium-catalyzed asymmetric hydrogenation of several alkenes and in the ruthenium-catalyzed hydrogenation of two... |
|
|
Bis-(2,5-diphenylphospholanes) with sp2 carbon linkers: synthesis and application in asymmetric hydrogenation.
J. Org. Chem. 73(3) , 775-84, (2008) Four chiral diphosphine ligands consisting of bis(2,5-diphenylphospholan-1-yl) groups connected by the sp(2) carbon linkers 2,3-quinoxaline ((S,S)-Ph-Quinox), 2,3-pyrazine ((S,S)-Ph-Pyrazine), maleic anhydride ((S,S)-Ph-MalPhos), and 1,1'-ferrocene ((S,S)-Ph-... |
|
|
Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi.
Bioresour. Technol. 99(11) , 5145-9, (2008) In this study, the antifungal activities of cinnamaldehyde and eugenol congeners against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus were evaluated and the relationships between the antifungal activity and the chemical struct... |
|
|
Total synthesis and biological evaluation of 22-hydroxyacuminatine.
J. Med. Chem. 49(4) , 1408-12, (2006) A total synthesis of 22-hydroxyacuminatine, a cytotoxic alkaloid isolated from Camptotheca acuminata, is reported. The key step in the synthesis involves the reaction of 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline with a brominated phthalide to generate a substitu... |