O-Benzylhydroxylamine hydrochloride
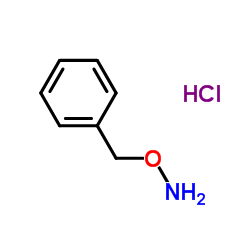
O-Benzylhydroxylamine hydrochloride structure
|
Common Name | O-Benzylhydroxylamine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2687-43-6 | Molecular Weight | 159.61 | |
| Density | N/A | Boiling Point | 237.5ºC at 760 mmHg | |
| Molecular Formula | C7H10ClNO | Melting Point | 238 °C (subl.)(lit.) | |
| MSDS | USA | Flash Point | 113.1ºC | |
|
Determination of carbohydrates in tobacco by pressurized liquid extraction combined with a novel ultrasound-assisted dispersive liquid-liquid microextraction method.
Anal. Chim. Acta 882 , 90-100, (2015) A novel derivatization-ultrasonic assisted-dispersive liquid-liquid microextraction (UA-DLLME) method for the simultaneous determination of 11 main carbohydrates in tobacco has been developed. The combined method involves pressurized liquid extraction (PLE), ... |
|
|
Capillary gas-chromatographic analysis of monosaccharides: improvements and comparisons using trifluoroacetylation and trimethylsilylation of sugar O-benzyl- and O-methyl-oximes.
Carbohydr. Res. 194 , 1, (1989) Two new procedures for the gas-chromatographic analysis of monosaccharides are reported. One involves derivatization of the sugars by reaction with O-benzylhydroxylamine followed by trifluoroacetylation with N-methylbis(trifluoroacetamide) and chromatography ... |
|
|
Thermodynamic analysis of the binding of aromatic hydroxamic acid analogues to ferric horseradish peroxidase.
Biochemistry 40(46) , 13980-9, (2001) Peroxidases typically bind their reducing substrates weakly, with K(d) values in the millimolar range. The binding of benzhydroxamic acid (BHA) to ferric horseradish peroxidase isoenzyme C (HRPC) [K(d) = 2.4 microM; Schonbaum, G. R. (1973) J. Biol. Chem. 248,... |
|
|
Analytical derivatizations of volatile and hydrophilic carbonyls from aqueous matrix onto a solid phase of a polystyrene-divinylbenzene macroreticular resin.
J. Chromatogr. B. Biomed. Sci. Appl. 694(2) , 289-96, (1997) Extraction and derivatization of carbonyls to benzyloximes, pentafluorobenzyloximes or 2,4-dinitrophenylhydrazones is simplified and reaction times are substantially reduced by simultaneous sorption and derivatization from aqueous solution onto a solid phase.... |
|
|
[Thermographic analysis of precipitates formed by the interaction of active ingredients and additives].
Acta Pharm. Hung. 53(6) , 268-72, (1983)
|
|
|
Regiospecific solid-phase strategy to N7-substituted purines and its application to 8-azapurines and [I]-condensed purines.
J. Comb. Chem. 9(5) , 804-10, (2007) A highly regioselective and traceless solid-phase route to 1,7,8-trisubstituted purines has been developed. This methodology could be extended to the preparation of 8-azapurines and [i]-condensed purines. A representative set of 17 purines, azapurines, and [i... |
|
|
A common mechanism of action for the N-glycosylase activity of DNA N-glycosylase/AP lyases from E. coli and T4.
Mutat. Res. 364(3) , 193-207, (1996) Duplex oligonucleotides containing the base lesion analogs, O-methylhydroxylamine- and O-benzylhydroxylamine-modified abasic (AP) sites, were substrates for the DNA N-glycosylases endonuclease III, formamidopyrimidine DNA N-glycosylase and T4 endonuclease V. ... |
|
|
NMR investigations on boron complexes in the conjugate addition on alpha,beta-unsaturated imides.
Org. Lett. 3(8) , 1165-7, (2001) [reaction: see text]. The 1,4-addition of O-benzylhydroxylamine to alpha,beta-unsaturated imide 1 in the presence of BF3.Et2O proceeds with the preferential attack of the nucleophile on the Cbeta-re face. To explain this unexpected reactivity 1H, 13C, and 11B... |
|
|
Gas chromatography of sample monocarbonyls in cigarette whole smoke as the benzyloxyamine derivatives.
J. Chromatogr. A. 202(2) , 255-61, (1980) A qualitative and semi-quantitative method was established for the investigation of low-molecular-weight volatile carbonyl compounds in cigarette whole smoke. The carbonyls were trapped on a silica gel "column" and eluted with water. The aqueous solution was ... |
|
|
Acrolein toxicity: Comparison with reactive oxygen species.
Biochem. Biophys. Res. Commun. 378 , 313-318, (2009) The toxicity of acrolein was compared with that of reactive oxygen species using a mouse mammary carcinoma FM3A cell culture system. Complete inhibition of cell growth was accomplished with 10 microM acrolein, 100 microM H(2)O(2), and 20 microM H(2)O(2) plus ... |