| Structure | Name/CAS No. | Articles |
|---|---|---|
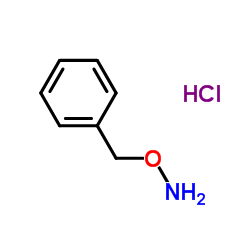 |
O-Benzylhydroxylamine hydrochloride
CAS:2687-43-6 |
G Cardillo, L Gentilucci, M Gianotti, A Tolomelli
Index: Org. Lett. 3(8) , 1165-7, (2001)
Full Text: HTML
[reaction: see text]. The 1,4-addition of O-benzylhydroxylamine to alpha,beta-unsaturated imide 1 in the presence of BF3.Et2O proceeds with the preferential attack of the nucleophile on the Cbeta-re face. To explain this unexpected reactivity 1H, 13C, and 11B NMR investigations have been carried out on the boron-imide complex, which show the presence of an S-cis imide chain conformation.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
O-Benzylhydroxylamine hydrochloride
CAS:2687-43-6 |
C7H10ClNO |
|
Determination of carbohydrates in tobacco by pressurized liq...
2015-07-02 [Anal. Chim. Acta 882 , 90-100, (2015)] |
|
Capillary gas-chromatographic analysis of monosaccharides: i...
1989-12-01 [Carbohydr. Res. 194 , 1, (1989)] |
|
Thermodynamic analysis of the binding of aromatic hydroxamic...
2001-11-20 [Biochemistry 40(46) , 13980-9, (2001)] |
|
Analytical derivatizations of volatile and hydrophilic carbo...
1997-07-04 [J. Chromatogr. B. Biomed. Sci. Appl. 694(2) , 289-96, (1997)] |
|
[Thermographic analysis of precipitates formed by the intera...
1983-11-01 [Acta Pharm. Hung. 53(6) , 268-72, (1983)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved