Sulpiride
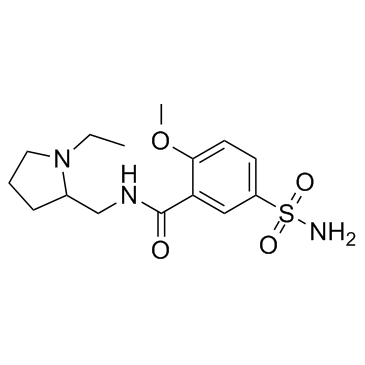
Sulpiride structure
|
Common Name | Sulpiride | ||
|---|---|---|---|---|
| CAS Number | 15676-16-1 | Molecular Weight | 341.426 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H23N3O4S | Melting Point | 180 - 185ºC | |
| MSDS | USA | Flash Point | N/A | |
|
Carbonic anhydrase inhibitors. Inhibition studies with anions and sulfonamides of a new cytosolic enzyme from the scleractinian coral Stylophora pistillata.
Bioorg. Med. Chem. Lett. 21 , 710-4, (2011) The catalytic activity and the inhibition of a new coral carbonic anhydrase (CA, EC 4.2.1.1), from the scleractinian coral Stylophora pistillata, STPCA-2, has been investigated. STPCA-2 has high catalytic activity for the physiological reaction being less sen... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
The Japanese toxicogenomics project: application of toxicogenomics.
Mol. Nutr. Food. Res. 54 , 218-27, (2010) Biotechnology advances have provided novel methods for the risk assessment of chemicals. The application of microarray technologies to toxicology, known as toxicogenomics, is becoming an accepted approach for identifying chemicals with potential safety proble... |
|
|
Calculating virtual log P in the alkane/water system (log P(N)(alk)) and its derived parameters deltalog P(N)(oct-alk) and log D(pH)(alk).
J. Med. Chem. 48 , 3269-79, (2005) Growing interest in the use of both the logarithm of the partition coefficient of the neutral species in the alkane/water system (log P(N)(alk)) and the difference between log P(N)(oct) (the logarithm of the partition coefficient of the neutral species in the... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Poisoning: fact or fiction?
Med. Leg. J. 80(Pt 4) , 127-48, (2012) Analytical toxicology is a complex discipline. Simply detecting a poison in a biological sample does not necessarily mean that the individual from whom the sample was obtained had been poisoned. An analysis can prove exposure and perhaps give an indication of... |
|
|
Neural computational prediction of oral drug absorption based on CODES 2D descriptors.
Eur. J. Med. Chem. 45 , 930-40, (2010) A neural model based on a numerical molecular representation using CODES program to predict oral absorption of any structure is described. This model predicts both high and low-absorbed compounds with a global accuracy level of 74%. CODES/ANN methodology show... |
|
|
Computational modeling of novel inhibitors targeting the Akt pleckstrin homology domain.
Bioorg. Med. Chem. 17 , 6983-92, (2009) Computational modeling continues to play an important role in novel therapeutics discovery and development. In this study, we have investigated the use of in silico approaches to develop inhibitors of the pleckstrin homology (PH) domain of AKT (protein kinase... |
|
|
Cloning, characterization and sulfonamide inhibition studies of an α-carbonic anhydrase from the living fossil sponge Astrosclera willeyana.
Bioorg. Med. Chem. 20 , 1403-10, (2012) The α-carbonic anhydrase (CA, EC 4.2.1.1) Astrosclerin-3 previously isolated from the living fossil sponge Astrosclera willeyana (Jackson et al., Science 2007, 316, 1893), was cloned, kinetically characterized and investigated for its inhibition properties wi... |
|
|
QSAR-based permeability model for drug-like compounds.
Bioorg. Med. Chem. 19 , 2615-24, (2011) A QSAR model was developed for predicting intestinal drug permeability, one of the most important parameters when evaluating compounds in drug discovery projects. First, a set of relevant properties for establishing a drug-like chemical space was applied to a... |