Sulpiride
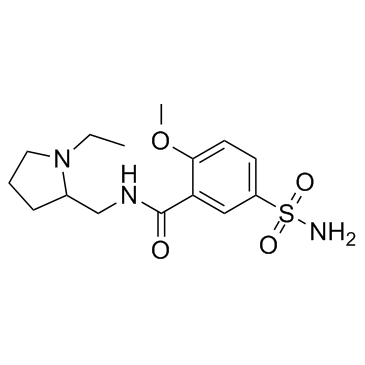
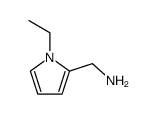
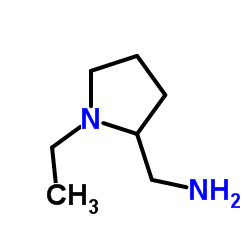
Sulpiride structure
|
Common Name | Sulpiride | ||
|---|---|---|---|---|
| CAS Number | 15676-16-1 | Molecular Weight | 341.426 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H23N3O4S | Melting Point | 180 - 185ºC | |
| MSDS | USA | Flash Point | N/A | |
Use of SulpirideSulpiride is a D2 receptor a antagonist, an atypical antipsychotic drug of the benzamide class, used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder, and sometimes used in low dosage to treat anxiety and mild depression. |
| Name | sulpiride |
|---|---|
| Synonym | More Synonyms |
| Description | Sulpiride is a D2 receptor a antagonist, an atypical antipsychotic drug of the benzamide class, used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder, and sometimes used in low dosage to treat anxiety and mild depression. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 180 - 185ºC |
| Molecular Formula | C15H23N3O4S |
| Molecular Weight | 341.426 |
| Exact Mass | 341.140930 |
| PSA | 110.11000 |
| LogP | 0.45 |
| Index of Refraction | 1.552 |
| Storage condition | 2-8°C |
| Water Solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 8.0 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~24%
Detail
|
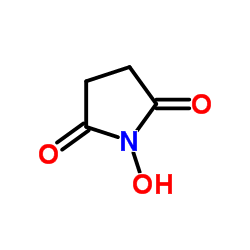
| Literature: Ogura; Haruo Patent: US4341707 A1, 1982 ; |
|
~% 
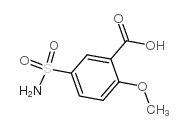
Sulpiride CAS#:15676-16-1 |
| Literature: US4242507 A1, ; |
|
~75% 
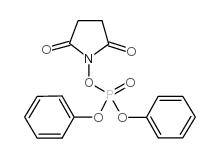
Sulpiride CAS#:15676-16-1 |
| Literature: Valenta, Vladimir; Protiva, Miroslav Collection of Czechoslovak Chemical Communications, 1987 , vol. 52, # 8 p. 2095 - 2106 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Carbonic anhydrase inhibitors. Inhibition studies with anions and sulfonamides of a new cytosolic enzyme from the scleractinian coral Stylophora pistillata.
Bioorg. Med. Chem. Lett. 21 , 710-4, (2011) The catalytic activity and the inhibition of a new coral carbonic anhydrase (CA, EC 4.2.1.1), from the scleractinian coral Stylophora pistillata, STPCA-2, has been investigated. STPCA-2 has high catal... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
The Japanese toxicogenomics project: application of toxicogenomics.
Mol. Nutr. Food. Res. 54 , 218-27, (2010) Biotechnology advances have provided novel methods for the risk assessment of chemicals. The application of microarray technologies to toxicology, known as toxicogenomics, is becoming an accepted appr... |
| 5-(aminosulfonyl)-N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxybenzamide |
| Benzamide, 5-(aminosulfonyl)-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxy- |
| Pyrikappl |
| sulpiridum |
| N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxy-5-sulfamoylbenzamide |
| SULPIRIDE |
| EINECS 239-753-7 |
| (S)-(-)-Sulpiride |
| N-[(1-Ethyl-2-pyrrolidinyl)methyl]-2-methoxy-5-sulfamoylbenzamide |
| sulpirida |
| N-[(1-Ethyl-2-pyrrolidinyl)methyl]-5-sulfamoyl-o-anisamide |
| (±)-5-(Aminosulfonyl)-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide |
| Synedil |
| MFCD00055061 |
| UNII:7MNE9M8287 |