4-hydroxyestrone
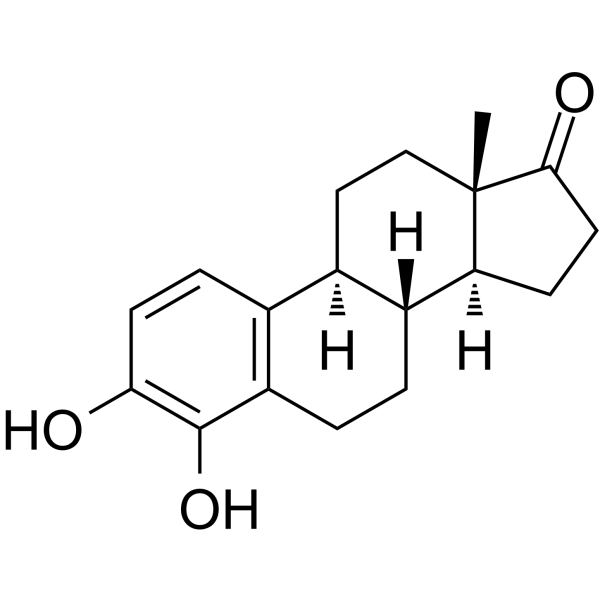
4-hydroxyestrone structure
|
Common Name | 4-hydroxyestrone | ||
|---|---|---|---|---|
| CAS Number | 3131-23-5 | Molecular Weight | 286.36500 | |
| Density | 1.241g/cm3 | Boiling Point | 468.2ºC at 760mmHg | |
| Molecular Formula | C18H22O3 | Melting Point | 260-263ºC | |
| MSDS | Chinese USA | Flash Point | 251.1ºC | |
|
Improved profiling of estrogen metabolites by orbitrap LC/MS.
Steroids 99 , 84-90, (2015) Estrogen metabolites are important biomarkers to evaluate cancer risks and metabolic diseases. Due to their low physiological levels, a sensitive and accurate method is required, especially for the quantitation of unconjugated forms of endogenous steroids and... |
|
|
Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens.
Chem. Res. Toxicol. 23(8) , 1365-73, (2010) Metabolic activation of estrogens to catechols and further oxidation to highly reactive o-quinones generates DNA damage including apurinic/apyrimidinic (AP) sites. 4-Hydroxyequilenin (4-OHEN) is the major catechol metabolite of equine estrogens present in est... |
|
|
Menstrual cycle effects on urinary estrogen metabolites.
J. Clin. Endocrinol. Metab. 84(11) , 3914-8, (1999) Endogenous estrogen metabolism may play an important role in the pathogenesis of hormone-related cancers, most notably breast cancer. Despite the importance of estrogen metabolism, little is known about estrogen metabolite profiles during different phases of ... |
|
|
The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone.
J. Endocrinol. 183(1) , 91-9, (2004) Several investigators have suggested that certain hydroxylated metabolites of 17beta-estradiol (E2) are the proximate carcinogens that induce mammary carcinomas in estrogen-sensitive rodent models. The studies reported here were designed to examine the carcin... |
|
|
Synthesis of N-acetylcysteine conjugates of catechol estrogens.
Steroids 61(5) , 296-301, (1996) The synthesis of N-acetylcysteine conjugates of 2-hydroxyestrone (2-OHE1) and 4-hydroxyestrone (4-OHE1) is described. The reaction of estrone 2,3-quinone with N-acetylcysteine provided 2-OHE1 and its C-4 and C-1 thioether conjugates in a ratio of 1:1, while e... |
|
|
In vitro generation of peroxynitrite by 2- and 4-hydroxyestrogens in the presence of nitric oxide.
Chem. Res. Toxicol. 14(5) , 547-54, (2001) Estrogen metabolism is altered in most, if not all, breast cancer tumors. These alterations primarily lead to the formation of the catechol estrogen metabolites, 2- and 4-hydroxyestrogens, which can generate superoxide anion radicals (O(2)(*)(-)) through the ... |
|
|
The ability of four catechol estrogens of 17beta-estradiol and estrone to induce DNA adducts in Syrian hamster embryo fibroblasts.
Carcinogenesis 22(9) , 1505-10, (2001) Catechol estrogens are considered critical intermediates in estrogen-induced carcinogenesis. We demonstrated previously that 17beta-estradiol (E(2)), estrone (E(1)) and four of their catechol estrogens, 2- and 4-hydroxyestradiols (2- and 4-OHE(2)), and 2- and... |
|
|
Estrogen receptor-independent catechol estrogen binding activity: protein binding studies in wild-type, Estrogen receptor-alpha KO, and aromatase KO mice tissues.
Biochemistry 43(21) , 6698-708, (2004) Primary evidence for novel estrogen signaling pathways is based upon well-documented estrogenic responses not inhibited by estrogen receptor antagonists. In addition to 17beta-E2, the catechol estrogen 4-hydroxyestradiol (4OHE2) has been shown to elicit biolo... |
|
|
The catechol estrogen, 4-hydroxyestrone, has tissue-specific estrogen actions.
J. Endocrinol. 167(2) , 281-7, (2000) Recent data indicate that the catechol estrogen, 2-hydroxyestrone (2-OHE(1)), has no effect on any target tissue including bone, whereas 16 alpha-hydroxyestrone (16 alpha-OHE(1)) exerts tissue-selective estrogen agonist activity. The effect of the catechol es... |
|
|
Determination of catechol and guaiacol estrogens in urine by capillary gas chromatography/mass spectrometry.
Biomed. Chromatogr. 7(3) , 172-6, (1993) A gas chromatographic (GC)/mass spectrometric method for the simultaneous determination in urine of 2- and 4-hydroxyestrones and hydroxyestradiols, and their monomethyl ethers, is described. Separation of these catechol and guaiacol estrogens was achieved by ... |