4-hydroxyestrone
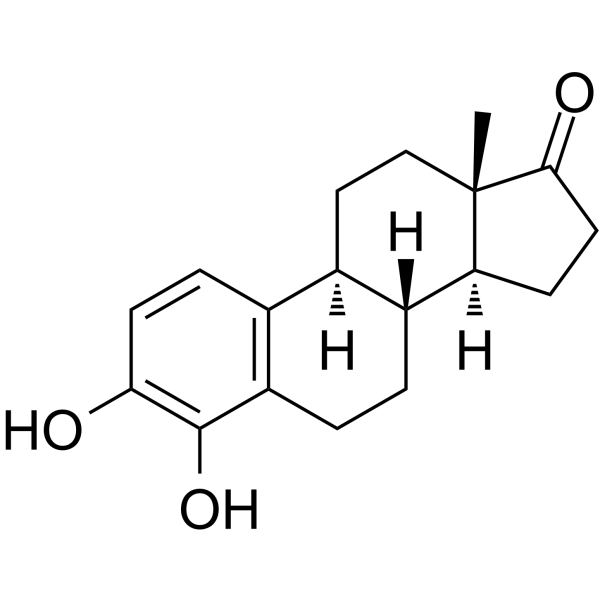
4-hydroxyestrone structure
|
Common Name | 4-hydroxyestrone | ||
|---|---|---|---|---|
| CAS Number | 3131-23-5 | Molecular Weight | 286.36500 | |
| Density | 1.241g/cm3 | Boiling Point | 468.2ºC at 760mmHg | |
| Molecular Formula | C18H22O3 | Melting Point | 260-263ºC | |
| MSDS | Chinese USA | Flash Point | 251.1ºC | |
Use of 4-hydroxyestrone4-Hydroxyestrone (4-OHE1), an estrone metabolite, has strong neuroprotective effect against oxidative neurotoxicity. 4-Hydroxyestrone increases cytoplasmic translocation of p53 resulting from SIRT1-mediated deacetylation of p53. 4-Hydroxyestrone has little estrogenic activity[1]. |
| Name | 4-hydroxyestrone |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Hydroxyestrone (4-OHE1), an estrone metabolite, has strong neuroprotective effect against oxidative neurotoxicity. 4-Hydroxyestrone increases cytoplasmic translocation of p53 resulting from SIRT1-mediated deacetylation of p53. 4-Hydroxyestrone has little estrogenic activity[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.241g/cm3 |
|---|---|
| Boiling Point | 468.2ºC at 760mmHg |
| Melting Point | 260-263ºC |
| Molecular Formula | C18H22O3 |
| Molecular Weight | 286.36500 |
| Flash Point | 251.1ºC |
| Exact Mass | 286.15700 |
| PSA | 57.53000 |
| LogP | 3.52300 |
| Index of Refraction | 1.61 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | KG7749500 |
| Precursor 9 | |
|---|---|
| DownStream 4 | |
|
Improved profiling of estrogen metabolites by orbitrap LC/MS.
Steroids 99 , 84-90, (2015) Estrogen metabolites are important biomarkers to evaluate cancer risks and metabolic diseases. Due to their low physiological levels, a sensitive and accurate method is required, especially for the qu... |
|
|
Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens.
Chem. Res. Toxicol. 23(8) , 1365-73, (2010) Metabolic activation of estrogens to catechols and further oxidation to highly reactive o-quinones generates DNA damage including apurinic/apyrimidinic (AP) sites. 4-Hydroxyequilenin (4-OHEN) is the m... |
|
|
Menstrual cycle effects on urinary estrogen metabolites.
J. Clin. Endocrinol. Metab. 84(11) , 3914-8, (1999) Endogenous estrogen metabolism may play an important role in the pathogenesis of hormone-related cancers, most notably breast cancer. Despite the importance of estrogen metabolism, little is known abo... |
| 4-Hydroxy Estrone |
| MFCD00079358 |
| (8R,9S,13S,14S)-3,4-dihydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one |
| 4-HydroxyEstrone |
 CAS#:53-16-7
CAS#:53-16-7 CAS#:210096-74-5
CAS#:210096-74-5 CAS#:14984-42-0
CAS#:14984-42-0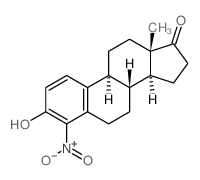 CAS#:5976-74-9
CAS#:5976-74-9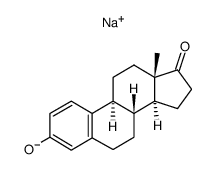 CAS#:74040-94-1
CAS#:74040-94-1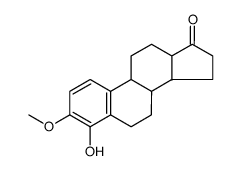 CAS#:5976-62-5
CAS#:5976-62-5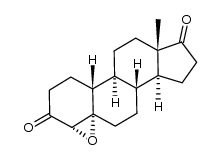 CAS#:210096-72-3
CAS#:210096-72-3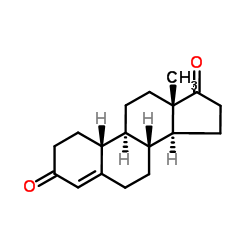 CAS#:734-32-7
CAS#:734-32-7 CAS#:81319-72-4
CAS#:81319-72-4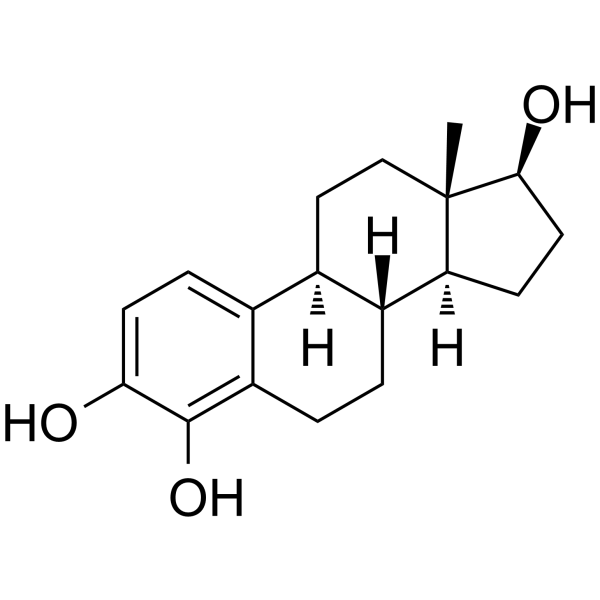 CAS#:5976-61-4
CAS#:5976-61-4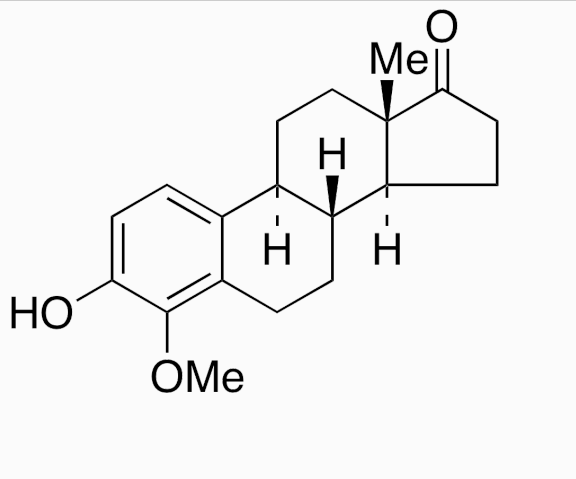 CAS#:58562-33-7
CAS#:58562-33-7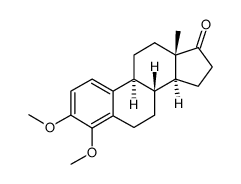 CAS#:26624-38-4
CAS#:26624-38-4